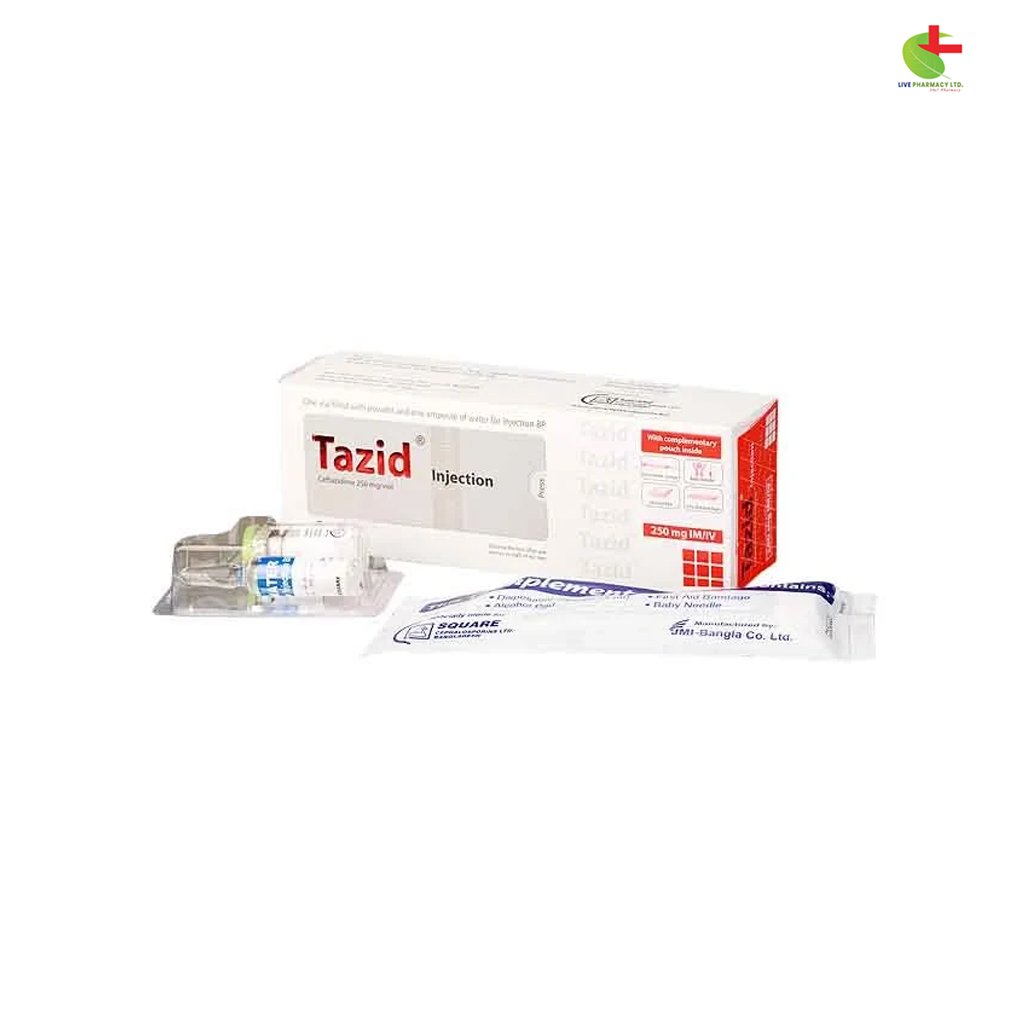
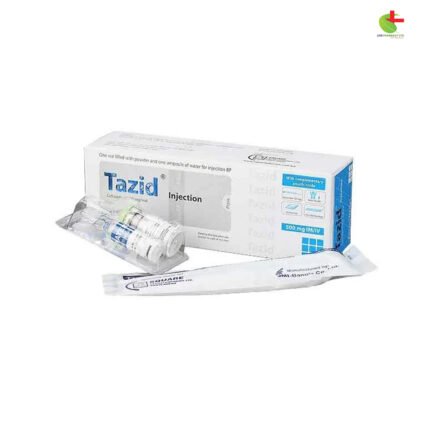
Tazid 250mg IV/IM
85.26৳ Piece
- Tazid Injection, a product of Square Pharmaceuticals PLC, is a potent antibiotic used for treating a variety of infections caused by susceptible organisms.
- Its broad-spectrum action makes it effective against lower respiratory tract infections, skin and skin structure infections, urinary tract infections, and more.
- With Ceftazidime as its active ingredient, it inhibits enzymes responsible for bacterial cell-wall synthesis, demonstrating bactericidal action.
- Careful dosage adjustments are recommended based on infection severity, patient factors, and renal function.
- Stored below 25°C, protected from light and moisture, Tazid Injection ensures stability and efficacy.
 Brand
Brand
|
Square Pharmaceuticals PLC |
|---|---|
 Generics
Generics
|
Ceftazidime Pentahydrate |
Indications
Tazid Injection is indicated for treating infections caused by susceptible strains of designated organisms in various diseases:
- Lower Respiratory Tract Infections, including pneumonia.
- Skin and Skin Structure Infections.
- Urinary Tract Infections, both complicated and uncomplicated.
- Bacterial Septicemia.
- Bone and Joint Infections.
- Gynecologic Infections.
- Intraabdominal Infections.
- Central Nervous System Infections.
Pharmacology
Tazid (Ceftazidime) is a broad-spectrum beta-lactam antibiotic, bactericidal in action, inhibiting enzymes responsible for cell-wall synthesis. It’s highly stable against clinically important beta-lactamases, active against gram-negative and gram-positive organisms.
Dosage
Dosage depends on infection severity, sensitivity, patient age, weight, and renal function. For adults, the usual dosage is 1 gram administered intravenously or intramuscularly every 8 to 12 hours.
Administration
Tazid can be given intravenously or by deep IM injection. It should be reconstituted with Sterile Water for Injection before administration.
Contraindications
Tazid is contraindicated in patients hypersensitive to Ceftazidime or cephalosporin antibiotics.
Side Effects
Common side effects include local reactions following IV injection, allergic reactions, and gastrointestinal symptoms. Central nervous system reactions such as headache and dizziness may occur.
Pregnancy & Lactation
Safety during pregnancy and lactation is not well-established, hence use should be considered if clearly needed.
Precautions & Warnings
Dosage adjustments are required for patients with renal insufficiency. Caution is advised in individuals with a history of gastrointestinal disease.
Storage Conditions
Store below 25°C, protected from light and moisture. Reconstituted solutions are stable for up to 24 hours if stored between 2°-8°C.
Therapeutic Class
Third generation Cephalosporins.
Reconstitution
For single-dose vials, add the specified amount of Sterile Water for Injection as per the dosage requirement.










Reviews
There are no reviews yet.