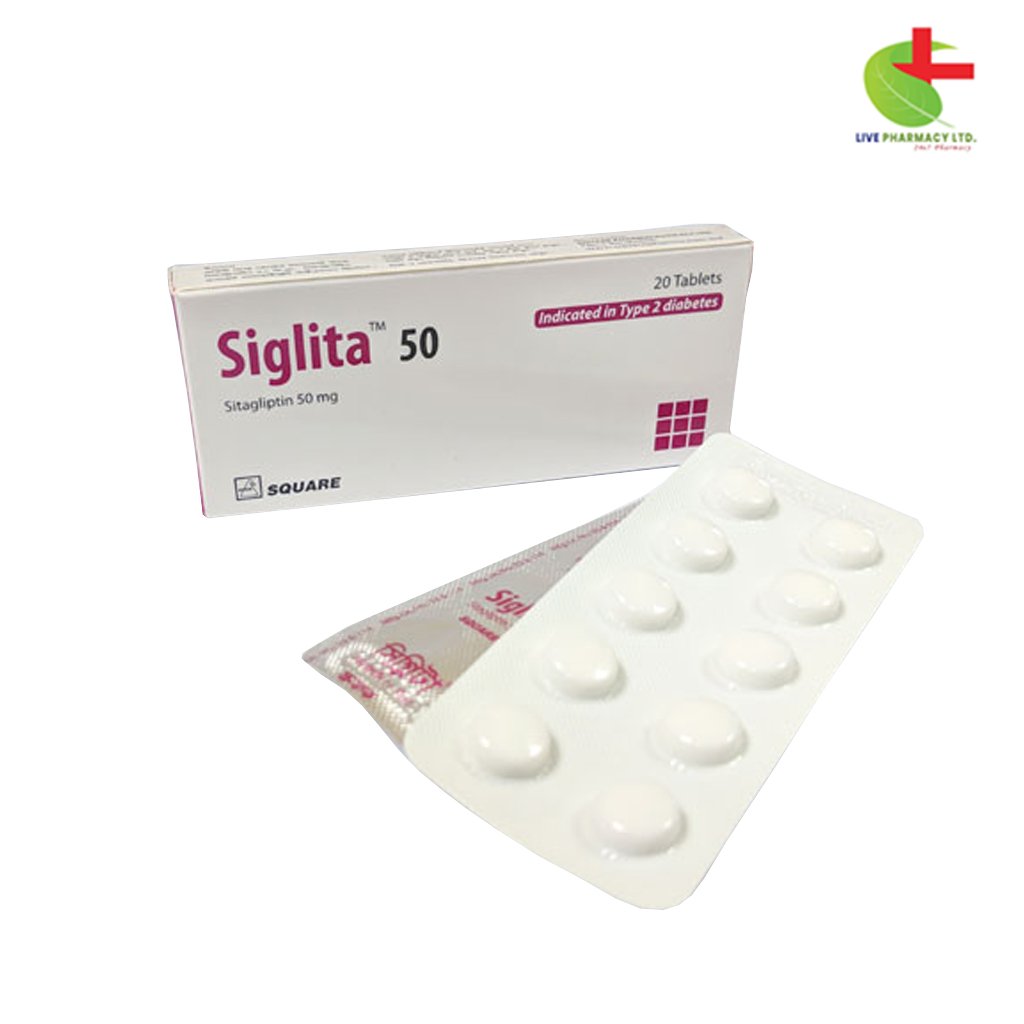
Siglita 50
130.00৳ Strip
- Trusted provider of Siglita for adults managing type 2 diabetes mellitus
- Enhances glycemic control through adjunct use with diet and exercise
- DPP-4 inhibitor that prolongs incretin hormone action
- Ensures safe dispensation under licensed healthcare guidance
 Brand
Brand
|
Square Pharmaceuticals PLC |
|---|---|
 Generics
Generics
|
Sitagliptin |
Indications
Monotherapy and Combination Therapy: Siglita serves as an adjunct to diet and exercise to enhance glycemic control in adults diagnosed with type 2 diabetes mellitus.
Important Usage Considerations: Siglita is not suitable for type 1 diabetes treatment or diabetic ketoacidosis management, as it is ineffective in these conditions. Its use has not been studied in patients with a history of pancreatitis, and whether these patients face elevated pancreatitis risk while using Siglita remains unknown.
Pharmacology
Sitagliptin, a DPP-4 inhibitor, is believed to enhance glycemic control in type 2 diabetes by inhibiting the breakdown of incretin hormones. By extending the activity of these hormones, such as GLP-1 and GIP, Sitagliptin increases insulin release and reduces glucagon secretion in a glucose-dependent manner. Sitagliptin selectively targets DPP-4 without affecting DPP-8 or DPP-9 activities at therapeutic doses.
Dosage & Administration
The recommended dosage of sitagliptin is either 50 mg twice daily or 100 mg once daily, with or without food.
Interactions
Siglita has negligible effects on the pharmacokinetics of metformin, glyburide, simvastatin, rosiglitazone, warfarin, or oral contraceptives.
Contraindications
Siglita should not be used in individuals with a history of serious hypersensitivity reactions to sitagliptin, such as anaphylaxis or angioedema.
Side Effects
Common adverse reactions may include headache, upper respiratory tract infections, and nasopharyngitis. Hypoglycemia may occur when Siglita is used in combination with sulfonylureas or insulin.
Pregnancy & Lactation
Sitagliptin is classified as Pregnancy Category B. Caution is advised when administering Siglita during pregnancy or lactation due to its secretion into the milk of lactating rats. The excretion of sitagliptin in human milk is unknown, necessitating careful consideration before use in nursing women.
Precautions & Warnings
Immediate discontinuation of Siglita is recommended if pancreatitis is suspected. Dose adjustments are advised in patients with renal insufficiency and when used concurrently with medications that induce hypoglycemia. Serious hypersensitivity reactions have been reported post-marketing.
Use in Special Populations
Safety and efficacy of Siglita have not been established in pediatric patients under 18 years old. Elderly patients, particularly those with renal impairment, should undergo careful dose selection and renal function assessment prior to initiation.
Overdose Effects
In clinical trials, doses up to 800 mg of Siglita in healthy subjects demonstrated no clinically significant adverse effects. Supportive measures are recommended in cases of overdose, including gastrointestinal decontamination and clinical monitoring.
Therapeutic Class
Dipeptidyl Peptidase-4 (DPP-4) inhibitor
Storage Conditions
Store Siglita below 25°C in a dry place away from light. Ensure it is kept out of reach of children and used before the expiration date. Dispense only under the prescription of a registered physician.





Reviews
There are no reviews yet.