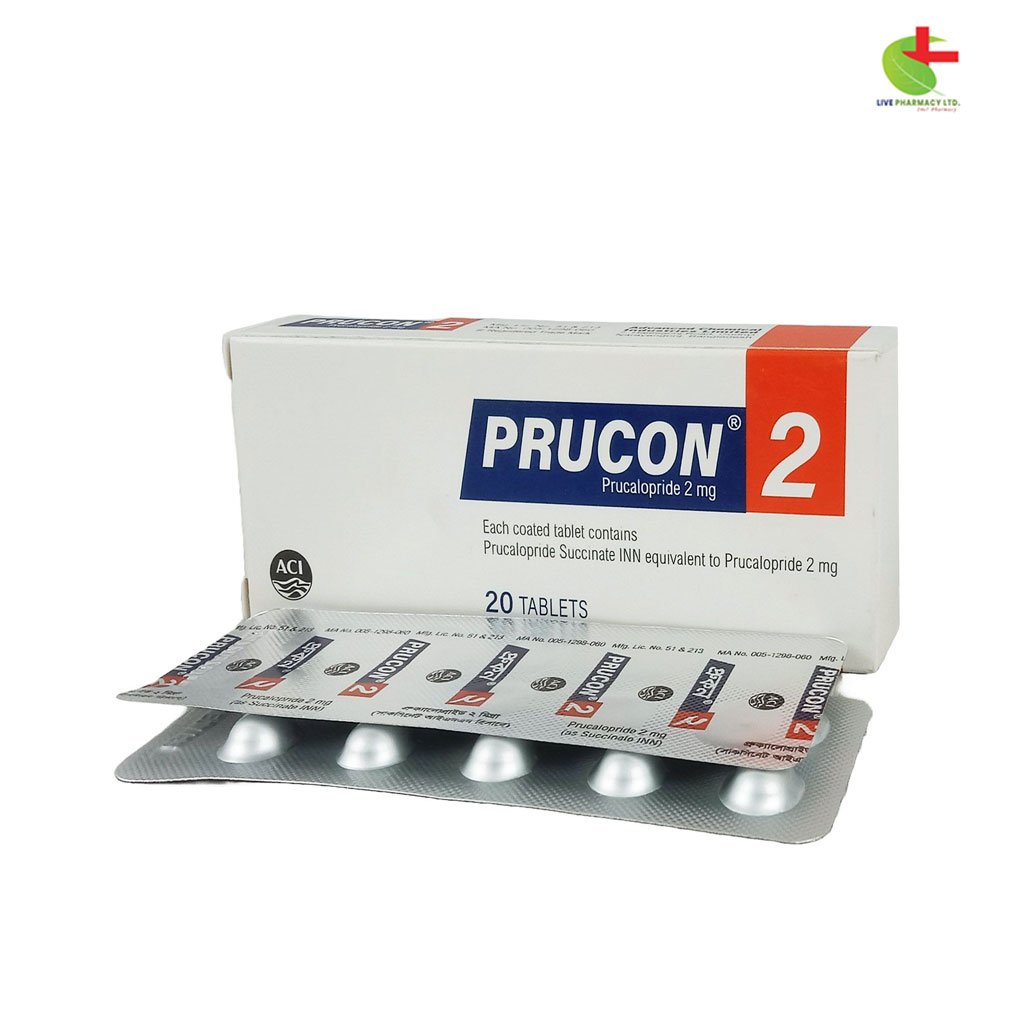
Prucon 2
240.00৳ Strip(10)
- Prucon Tablet is used to treat chronic constipation in adults when other laxatives are ineffective.
- It works by stimulating 5-HT4 receptors in the gastrointestinal tract to promote bowel motility.
- Recommended dosage is 2 mg once daily for adults, with adjustments for older adults and those with renal or hepatic impairment.
- Common side effects include headache, nausea, and diarrhea, typically resolving after a few days of use.
 Brand
Brand
|
ACI Limited |
|---|---|
 Generics
Generics
|
Prucalopride Succinate |
 Type
Type
|
Tablet |
Indications
Prucon tablet is recommended for the relief of chronic constipation symptoms in adults where other laxatives have not provided sufficient improvement.
Always consult a registered healthcare professional before using this medication.
Pharmacology
Prucalopride works as a targeted 5-HT4 receptor agonist, without affecting the hERG channel or 5-HT1 receptors, significantly reducing the cardiovascular risks associated with similar medications. These receptors are located throughout the gastrointestinal system, including in smooth muscle cells, enterochromaffin cells, and the myenteric plexus. When activated, they trigger the release of acetylcholine, the primary excitatory neurotransmitter in the GI tract, enhancing bowel motility. By interacting with 5-HT4 receptors, prucalopride stimulates muscle contractions in the colon, promoting the movement of digested contents.
Dosage & Administration
- Adults: Take 2 mg once daily, with or without food, at any time of the day. Exceeding 2 mg per day is not expected to increase its effectiveness due to its specific mechanism of action.
- Older Adults: Begin with 1 mg once daily, which may be increased to 2 mg if necessary.
- Children: Not recommended for use in children or adolescents under the age of 18.
Use as per the guidance of a registered healthcare professional.
Drug Interactions
Prucon shows minimal interaction with other drugs. Therapeutic doses of prucalopride are not likely to influence the metabolism of co-administered medications mediated by CYP enzymes. Although prucalopride may slightly interact with P-glycoprotein (P-gp), it does not inhibit P-gp at therapeutic levels.
Studies indicate that when combined with ketoconazole (a strong CYP3A4 and P-gp inhibitor), prucalopride’s exposure increases by about 40%, though this effect is not clinically significant. Similar interactions may occur with other potent P-gp inhibitors like verapamil, cyclosporine, and quinidine. No significant changes in the pharmacokinetics of warfarin, digoxin, alcohol, paroxetine, or oral contraceptives have been observed in clinical trials.
Contraindications
Prucon should not be used by individuals who are allergic to prucalopride or any of its ingredients. It is also contraindicated in patients with renal failure requiring dialysis.
Side Effects
Common side effects of Prucon include headache (17.8%) and gastrointestinal issues such as abdominal pain, nausea, and diarrhea. These symptoms typically subside within a few days of continued use. Most side effects are mild to moderate in nature.
Pregnancy & Lactation
Prucalopride is not recommended during pregnancy. Women of childbearing potential should use effective contraception while undergoing treatment. While animal studies have not shown harmful effects on pregnancy or fetal development, there is insufficient human data to confirm its safety during breastfeeding. Therefore, it is not advised to use prucalopride while breastfeeding.
Precautions & Warnings
- Renal Impairment: Since prucalopride is primarily eliminated through the kidneys, a reduced dose of 1 mg daily is recommended for patients with severe renal impairment (GFR <30 ml/min/1.73 m²).
- Hepatic Impairment: Caution should be exercised in patients with severe liver impairment (Child-Pugh class C). Start with 1 mg once daily, which may be increased to 2 mg if well tolerated. No dose adjustment is necessary for mild to moderate hepatic impairment.
- Diarrhea and Contraceptives: In cases of severe diarrhea, the effectiveness of oral contraceptives may be reduced. Consider using an additional contraceptive method to prevent failure.
- Lactose Content: This medication contains lactose and should not be used by individuals with rare hereditary issues such as galactose intolerance, lactase deficiency, or glucose-galactose malabsorption.
Use in Special Populations
- Renal Impairment: For those with severe renal impairment (GFR <30 ml/min/1.73 m²), the recommended dose is 1 mg daily. No adjustment is required for those with mild to moderate renal issues.
- Hepatic Impairment: Patients with severe hepatic impairment (Child-Pugh class C) should begin with 1 mg daily, with the option to increase to 2 mg based on tolerability. No dose modification is necessary for mild to moderate hepatic impairment.
Overdose Effects
An overdose of prucalopride may intensify its known effects, leading to symptoms such as headache, nausea, and diarrhea. No specific antidote is available, so treatment should be symptomatic. If significant fluid loss occurs due to diarrhea or vomiting, electrolyte disturbances may need correction.
Therapeutic Class
Osmotic laxatives.
Storage Conditions
Store at room temperature, below 30°C. Keep the desiccant in place and store in the original bottle.










Reviews
There are no reviews yet.