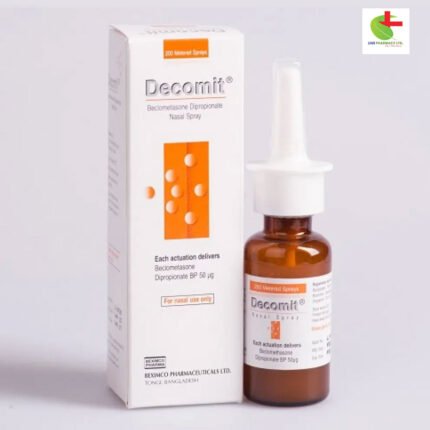
Fixonase Spray
275.00৳ Nasal Spray
- Fixonase is a synthetic, fluorinated corticosteroid used to treat allergic rhinitis and asthma.
- The nasal spray is effective for seasonal and perennial allergic rhinitis in patients aged 2 years and older.
- The inhalation powder serves as a daily maintenance therapy for asthma in patients aged 5 years and above.
- It provides potent anti-inflammatory effects by targeting multiple cells and mediators involved in inflammation.
 Brand
Brand
|
Beximco Pharmaceuticals Ltd |
|---|---|
 Generics
Generics
|
Fluticasone Furoate |
 Type
Type
|
Nasal Spray |
Indications
Fixonase Nasal Spray is prescribed for alleviating symptoms of both seasonal and perennial allergic rhinitis in patients aged 2 years and above.
Fixonase Inhalation Powder is used as a daily maintenance treatment for asthma, providing long-term prevention in patients aged 5 years and older. It is not suitable for immediate relief of acute bronchospasm.
Description
Fixonase is a synthetic, fluorinated corticosteroid known for its anti-inflammatory properties. The nasal spray consists of a microfine Fixonase suspension for localized administration through a metered, atomizing spray pump. The pump must be primed before initial use or if left unused for a week or longer. Each bottle delivers 120 precise doses. After 120 sprays, the amount dispensed may vary, and the bottle should be discarded.
Pharmacology
Fluticasone Furoate, a synthetic trifluorinated corticosteroid, exhibits strong anti-inflammatory effects. Like other corticosteroids, it interacts with various cell types (e.g., mast cells, neutrophils, eosinophils) and mediators (e.g., histamine, cytokines, eicosanoids) that are key players in inflammatory responses.
Dosage
Fluticasone Furoate Nasal Spray:
- Adults & Children 12 years and older: 2 sprays in each nostril once daily. If necessary, 2 sprays twice daily, but not exceeding 4 sprays in total.
- Children aged 2-11 years: 1 spray in each nostril once daily. Consistent use is essential for effectiveness.
- Children under 2 years: Not recommended, as no data supports its use in this age group.
Fluticasone Furoate Inhalation Capsule:
- Adults and Adolescents 12 years and older: Administer 1 inhalation daily. The starting dose should reflect asthma severity, with 100 mcg being typical for those not on corticosteroids. After 2 weeks, the dose can be increased to 200 mcg if necessary.
- Children aged 5-11 years: 50 mcg inhaled once daily.
Administration
How to Use Fixonase Nasal Spray:
- Shake the bottle and remove the dust cover.
- Press the pump until a fine spray appears (if first-time use or after a week of non-use).
- Clear nostrils by gently blowing the nose.
- Insert the applicator, press, and inhale through the nose. Breathe out through the mouth.
- Clean the spray weekly by rinsing the applicator and dust cover in warm water, air-drying, and reassembling.
Interactions
Strong inhibitors of cytochrome P450 3A4 (CYP3A4), such as ritonavir and ketoconazole, can elevate Fixonase levels. Co-administration with these drugs is not recommended.
Special Instructions
The device must be primed before first use and after extended non-use (30+ days). Shake vigorously before each use and press the button firmly to dispense.
Contraindications
Fixonase is contraindicated in patients with known hypersensitivity to any of its components.
Side Effects
Fixonase nasal spray typically has fewer systemic side effects due to minimal absorption. Possible nasal side effects include dryness and nosebleeds. The inhalation capsule may cause nasopharyngitis, bronchitis, respiratory infections, and headaches in adults and adolescents. Common pediatric side effects include pharyngitis and viral infections.
Pregnancy & Lactation
- Pregnancy: Fluticasone Furoate is a Category C drug. Use only if the benefits outweigh risks to the fetus.
- Lactation: It is unclear if Fluticasone Furoate passes into breast milk, but caution is advised.
Precautions & Warnings
Rarely, allergic reactions such as hypersensitivity or contact dermatitis may occur. Use cautiously in patients with cataracts, glaucoma, or elevated intraocular pressure. Higher-than-recommended doses should be avoided due to the potential for systemic side effects.
Special Populations
- Elderly: Dose adjustments should consider any pre-existing hepatic, renal, or cardiac conditions.
- Hepatic Impairment: Use cautiously in patients with severe liver issues.
- Renal Impairment: No dose adjustments are needed.
Therapeutic Class
Nasal Steroid Preparations
Storage Instructions
Store below 30°C. Avoid refrigeration and protect from light and moisture. Keep out of children’s reach.










Reviews
There are no reviews yet.