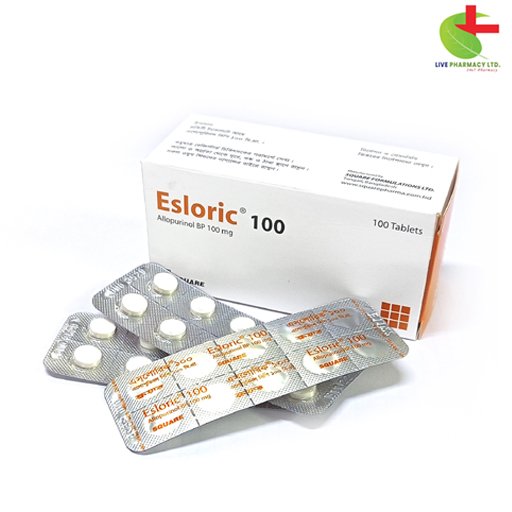
Esloric 100
40.30৳ Strip
- Discover Esloric: Your trusted solution for conditions like gouty arthritis, skin tophi, and nephrolithiasis.
- Effective Reduction: Esloric diminishes urate/uric acid formation, targeting the root cause of these ailments.
- Targeted Management: Specifically formulated to address renal stones related to adenine phosphoribosyltransferase deficiency.
- Potent Ingredients: Esloric contains Allopurinol, a powerful xanthine oxidase inhibitor for comprehensive relief.
- Healthcare Professional Approved: Trusted by experts, Esloric is a cornerstone in the treatment of gout and associated disorders.
 Brand
Brand
|
Square Pharmaceuticals PLC |
|---|---|
 Generics
Generics
|
Allopurinol |
Indications
Esloric serves to diminish the formation of urate/uric acid in conditions where their deposition has already occurred, such as gouty arthritis, skin tophi, and nephrolithiasis. It is also effective in managing 2,8-dihydroxyadenine (2,8-DHA) renal stones associated with deficient activity of adenine phosphoribosyltransferase. Additionally, Esloric is indicated for the management of recurrent mixed calcium oxalate renal stones in the presence of hyperuricosuria, when conventional measures like fluid intake and dietary adjustments have proven ineffective.
Pharmacology
Allopurinol, an orally administered xanthine oxidase inhibitor, modulates purine catabolism without interrupting purine biosynthesis. By inhibiting the biochemical reactions preceding uric acid formation, it reduces uric acid production. Structurally akin to hypoxanthine, Allopurinol impedes xanthine oxidase, the enzyme catalyzing the conversion of hypoxanthine to xanthine and xanthine to uric acid. With approximately 90% absorption from the gastrointestinal tract, peak plasma levels typically occur between 1.5 to 4.5 hours post-administration, boasting a plasma half-life of about 1 to 2 hours. Roughly 20% of ingested Allopurinol is excreted in the feces.
Dosage & Administration
For adults, Allopurinol initiation should commence at a low dosage, e.g., 100mg/day, to mitigate the risk of adverse reactions, with increments made only if the serum urate response proves inadequate. Caution is warranted in individuals with compromised renal function. Recommended dosage schedules include 100 to 200 mg daily for mild conditions, 300 to 600 mg daily for moderately severe conditions, and 700 to 900 mg daily for severe conditions.
In children under 15 years, dosing ranges from 10 to 20 mg/kg body weight/day, up to a maximum of 400 mg daily. Elderly patients should be administered the lowest effective dosage, considering the absence of specific data.
In cases of severe renal insufficiency, it may be advisable to administer less than 100 mg per day or single doses of 100mg at longer intervals than one day.
Interaction
Concurrent administration of Esloric with 6-mercaptopurine or azathioprine necessitates a reduction to one-quarter of the usual dose, given the prolongation of their activity owing to xanthine oxidase inhibition. Enhanced vigilance is imperative when using Esloric concomitantly with vidarabine to recognize potential toxic effects. Monitoring of theophylline levels is recommended when initiating or increasing Esloric therapy. Additionally, increased incidence of skin rash has been reported with concurrent use of ampicillin or amoxicillin with Esloric. Esloric may elevate plasma concentrations of ciclosporin during concomitant therapy.
Contraindications
Allopurinol tablet is contraindicated in patients with known hypersensitivity to allopurinol.
Side Effects
Side effects may include rashes, gastrointestinal disorders, malaise, headache, vertigo, drowsiness, visual and taste disturbances, hypertension, alopecia, hepatotoxicity, neuropathy, gynecomastia, and blood disorders.
Pregnancy & Lactation
The safety of Allopurinol in human pregnancy is inadequately established. Use during pregnancy is advisable only when no safer alternatives are available and when the benefits outweigh the risks. There is limited data regarding the effects of allopurinol or its metabolites on breastfed infants.
Precautions & Warnings
Immediate withdrawal of Esloric is advised upon manifestation of a skin rash or any evidence of sensitivity. Reduced doses are recommended for patients with hepatic or renal impairment. Caution is warranted in patients undergoing treatment for hypertension or cardiac insufficiency, as they may exhibit concurrent renal function impairment.
Overdose Effects
Reported cases of ingestion of up to 22.5 g of Esloric without adverse effects exist. Symptoms of overdose may include nausea, vomiting, diarrhea, and dizziness. Adequate hydration to maintain optimal diuresis facilitates the excretion of Esloric and its metabolites. Hemodialysis may be considered if necessary.
Therapeutic Class
Drugs used in Gout.
Storage Conditions
Store in a cool, dry place, shielded from light.





Reviews
There are no reviews yet.