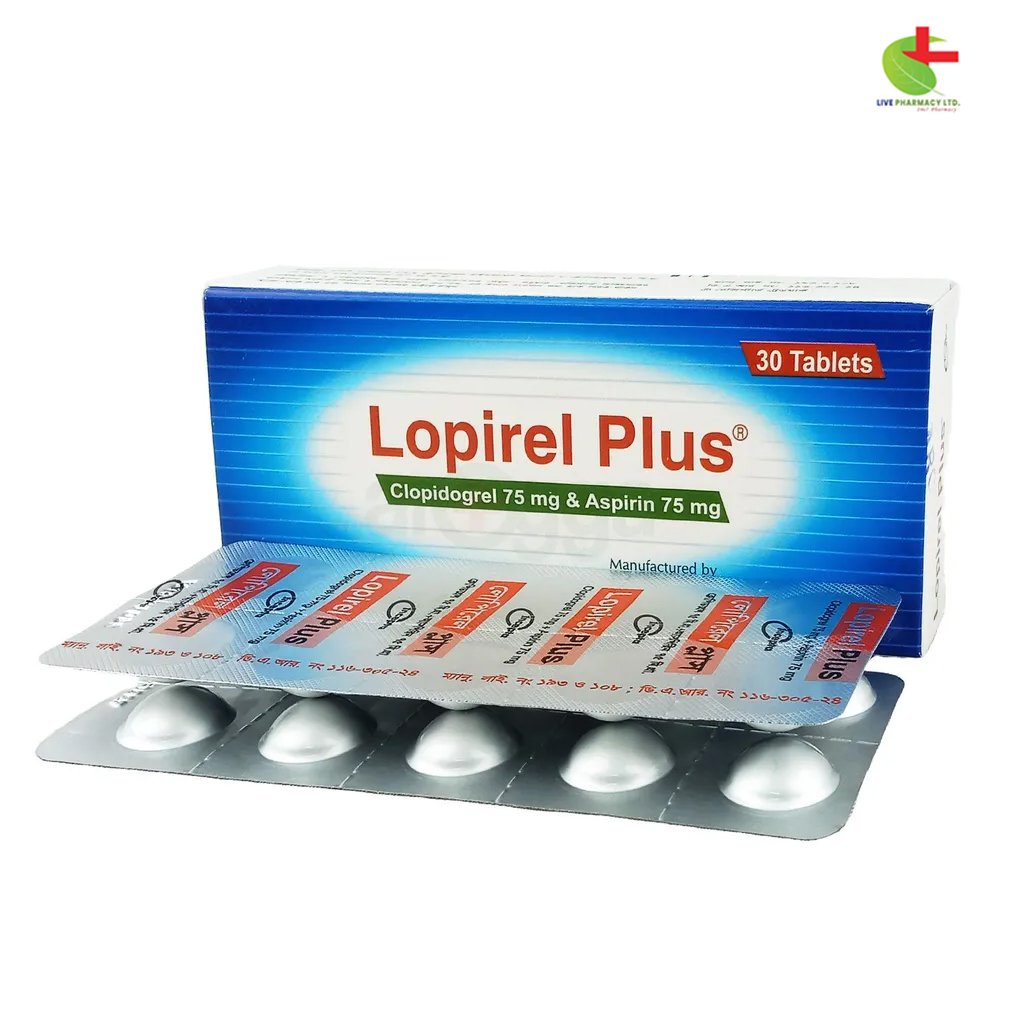
Lopirel Plus
120.00৳ Strip
- Lopirel Plus is an anti-platelet medication indicated for reducing the risk of myocardial infarction and stroke.
- Effective for patients with acute coronary syndrome, recent heart attacks, or peripheral arterial disease.
- Works by inhibiting platelet aggregation through its active metabolite.
- Administered once daily, it can be taken with or without food.
- Always consult a healthcare professional before use.
 Brand
Brand
|
Incepta Pharmaceuticals Ltd |
|---|---|
 Generics
Generics
|
Clopidogrel + Aspirin |
 Type
Type
|
Tablet |
Indications
Acute Coronary Syndrome (ACS): Lopirel 75 is indicated for reducing the risk of myocardial infarction (MI) and stroke in patients with both non-ST-segment elevation ACS—such as unstable angina (UA) and non-ST-elevation myocardial infarction (NSTEMI)—as well as acute ST-segment elevation myocardial infarction (STEMI).
Recent MI, Stroke, or Established Peripheral Arterial Disease: This medication is also suitable for patients with a history of recent MI, recent stroke, or established peripheral arterial disease to help decrease the likelihood of subsequent MI and stroke.
Please consult a registered healthcare professional before using this medication.
Pharmacology
Lopirel 75 acts as a prodrug, inhibiting platelet activation and aggregation by irreversibly binding to the P2Y12 ADP receptors on platelets. Noticeable inhibition of platelet aggregation can be detected within 2 hours after a single oral dose. With daily dosing of 75 mg, effective inhibition is achieved on the first day, stabilizing between Days 3 and 7.
Aspirin works by irreversibly inhibiting platelet cyclooxygenase, thereby reducing thromboxane A2—a potent inducer of platelet aggregation and vasoconstriction.
Pharmacokinetics: Following repeated doses of 75 mg, plasma levels of the parent compound remain low (below 0.00025 mg/L) after 2 hours. Clopidogrel is primarily metabolized in the liver, with the main circulating metabolite being a carboxylic acid derivative, which constitutes about 85% of drug-related compounds in the plasma. About 50% of the administered dose is excreted in urine, while 46% is eliminated through feces over five days. The half-life of the main metabolite is approximately 8 hours, and food does not significantly affect its bioavailability.
Absorption and Distribution: Clopidogrel is quickly absorbed, reaching peak plasma levels of the main metabolite about 1 hour after administration. The pharmacokinetics are linear across doses from 50 to 150 mg, with at least 50% absorption based on urinary excretion of metabolites. Clopidogrel and its metabolite show strong reversible binding to plasma proteins (98% and 94%, respectively).
Metabolism and Elimination: Clopidogrel rapidly converts to its carboxylic acid derivative, with glucuronide forms also detected in plasma and urine.
Dosage & Administration
The recommended dosage of Lopirel 75 is 75 mg once daily, with or without food. No dosage adjustments are necessary for elderly patients or those with renal conditions. For patients with acute coronary syndrome (unstable angina/non-Q-wave MI), initiate treatment with a 300 mg loading dose, followed by 75 mg daily. It is recommended to combine with Aspirin (75 mg-325 mg daily). Studies show that most patients with acute coronary syndrome also receive heparin treatment.
Please consult a registered healthcare professional before using this medication.
Interactions
- Aspirin: Does not alter Clopidogrel’s effect on ADP-induced platelet aggregation; however, it enhances Clopidogrel’s effect on collagen-induced platelet aggregation.
- Heparin: Co-administration does not require adjustments to the heparin dosage and does not affect Clopidogrel’s platelet aggregation inhibition.
- NSAIDs: Caution is advised when using NSAIDs with Clopidogrel due to increased risk of gastrointestinal bleeding.
- Warfarin: Safety during co-administration with Clopidogrel has not been established; use caution.
Contraindications
Lopirel 75 should not be used in individuals with known hypersensitivity to the drug or any of its components, or in cases of active bleeding conditions such as peptic ulcers or intracranial hemorrhage.
Side Effects
Lopirel 75 is generally well tolerated. Potential side effects include:
- Common: Bleeding, gastrointestinal discomfort, diarrhea, skin reactions.
- Rare: Acquired hemophilia, anemia, angioedema, joint pain, bone marrow disorders.
Pregnancy & Lactation
There are insufficient well-controlled studies regarding the use of Clopidogrel in pregnant women. It should be used during the first and second trimesters only when necessary and is contraindicated during the third trimester. The excretion of Clopidogrel in breast milk is unknown; however, since Aspirin is known to be excreted in breast milk, it is advised to discontinue the drug during breastfeeding.
Precautions & Warnings
- Lopirel 75 may prolong bleeding time.
- Thrombotic thrombocytopenic purpura (TTP) has been reported rarely following use.
- Reye’s syndrome may develop in children with chickenpox or flu-like symptoms; thus, caution is advised in pediatric patients.
- Hypersensitivity reactions, such as rash or angioedema, have been observed, particularly in patients with a history of sensitivity to thienopyridines.
Use in Special Populations
Lopirel 75 is not recommended for children under 12 unless benefits significantly outweigh risks. Aspirin may contribute to Reye’s syndrome in certain pediatric cases.
Overdose Effects
Overdose of Clopidogrel may lead to bleeding complications; platelet transfusions may help restore clotting ability. Moderate Aspirin intoxication can cause dizziness, headache, and gastrointestinal symptoms, which may require treatment such as vomiting or gastric lavage. Severe intoxication can lead to respiratory acidosis, hyperthermia, and dehydration, potentially necessitating hemodialysis.
Therapeutic Class
Anti-platelet Drugs
Storage Conditions
Store in a cool, dry place (below 30ºC), protected from light and moisture. Keep out of reach of children.










Reviews
There are no reviews yet.