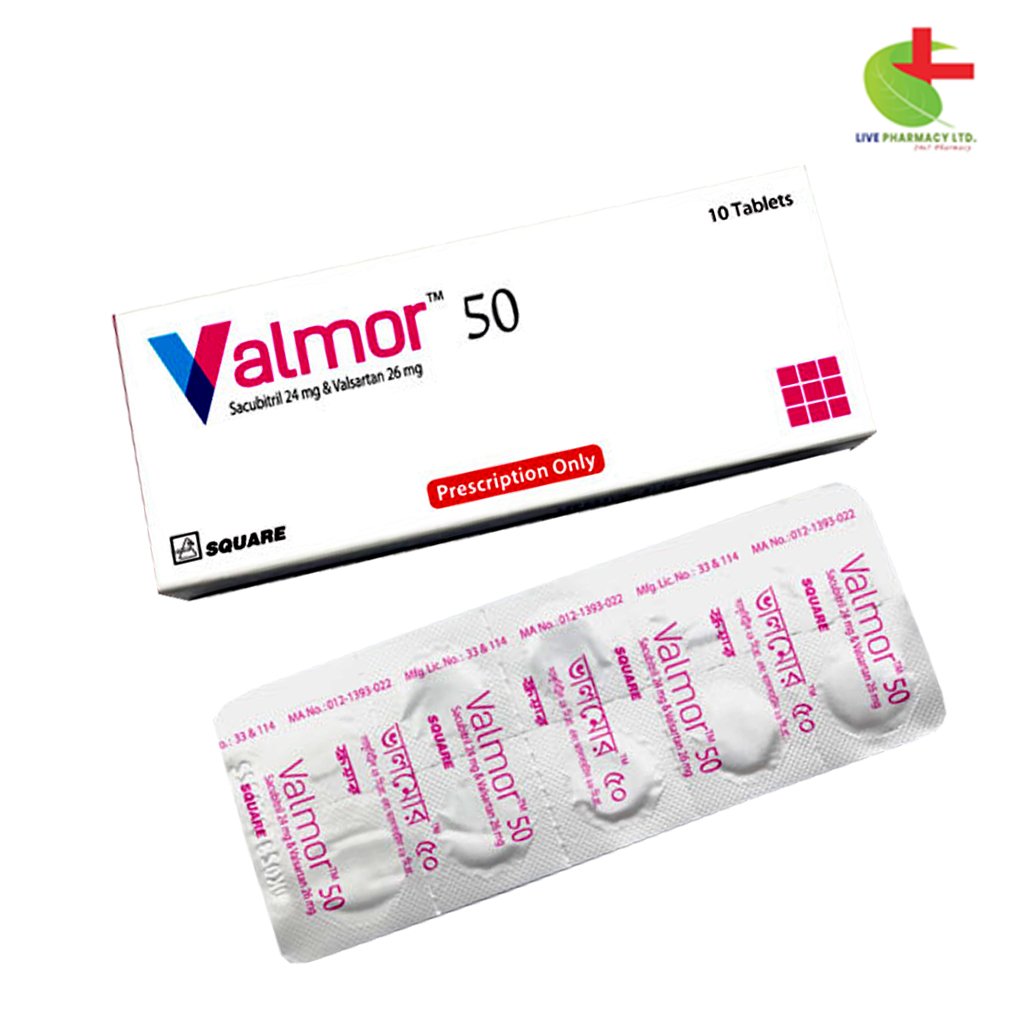
Valmor 50
450.00৳ Strip
- We provide a comprehensive range of pharmaceutical services to enhance health and well-being.
- Our team offers personalized care with a focus on quality and support.
- Services include medication management and health consultations.
- Experience trusted pharmacy care tailored to your needs.
 Brand
Brand
|
Square Pharmaceuticals PLC |
|---|---|
 Generics
Generics
|
Sacubitril + Valsartan |
 Type
Type
|
Tablet |
Indications
This medication is prescribed for:
- Reducing the risk of cardiovascular death and hospitalization for heart failure in patients with chronic heart failure (NYHA Class II-IV) and reduced ejection fraction.
- Treating symptomatic heart failure with systemic left ventricular systolic dysfunction in pediatric patients aged one year and older.
- Typically used alongside other heart failure therapies, replacing an angiotensin-converting enzyme inhibitor (ACEi) or another angiotensin II receptor blocker (ARB).
Pharmacology
Each tablet contains sacubitril, a neprilysin inhibitor, and valsartan, an angiotensin receptor blocker. Sacubitril inhibits neprilysin (neutral endopeptidase; NEP) via its active metabolite LBQ657, thereby increasing levels of peptides like natriuretic peptides that neprilysin degrades. Valsartan blocks the angiotensin II type-1 (AT1) receptor, reducing the effects of angiotensin II and inhibiting aldosterone release.
Dosage & Administration
For adult heart failure, start with 49/51 mg orally twice daily, then double the dose after 2 to 4 weeks to reach a maintenance dose of 97/103 mg twice daily, adjusting as tolerated.
Begin with 24/26 mg twice daily for:
- Patients not currently on an ACEi or ARB or previously taking a low dose of these agents.
- Patients with severe renal impairment.
- Patients with moderate hepatic impairment.
For pediatric heart failure, refer to Table 1 for dosing guidelines based on weight, adjusting every 2 weeks.
Interaction
Avoid using with an ACEi or aliskiren in patients with diabetes, and use cautiously with an ARB. May increase serum potassium levels with potassium-sparing diuretics and risk of renal impairment with NSAIDs. Increased risk of lithium toxicity with lithium.
Contraindications
Do not use in patients with hypersensitivity to any component or history of angioedema related to previous ACEi or ARB therapy. Avoid within 36 hours of switching to or from an ACE inhibitor or with aliskiren in diabetes.
Side Effects
Common side effects include angioedema, hypotension, impaired renal function, hyperkalemia, cough, and dizziness.
Pregnancy & Lactation
Safety not established in pediatric patients under 1 year. No significant pharmacokinetic differences observed in elderly or very elderly patients. Use with caution in moderate hepatic impairment; not recommended in severe hepatic impairment.
Precautions & Warnings
May cause angioedema; avoid in patients with a history of angioedema related to ACEi or ARB therapy. Monitor blood pressure and renal function closely. Periodically monitor serum potassium and adjust treatment as needed.
Use in Special Populations
Safety and effectiveness not established in pediatric patients. No dose adjustment necessary in mild to moderate renal impairment. Avoid use in severe renal impairment. Use with caution in moderate hepatic impairment; avoid in severe hepatic impairment.
Overdose Effects
Limited data available; manage symptoms of hypotension with symptomatic treatment. Unlikely to be removed by hemodialysis due to high protein binding.
Storage Conditions
Store below 30°C in a dry place, protected from moisture and out of children’s reach.






Reviews
There are no reviews yet.