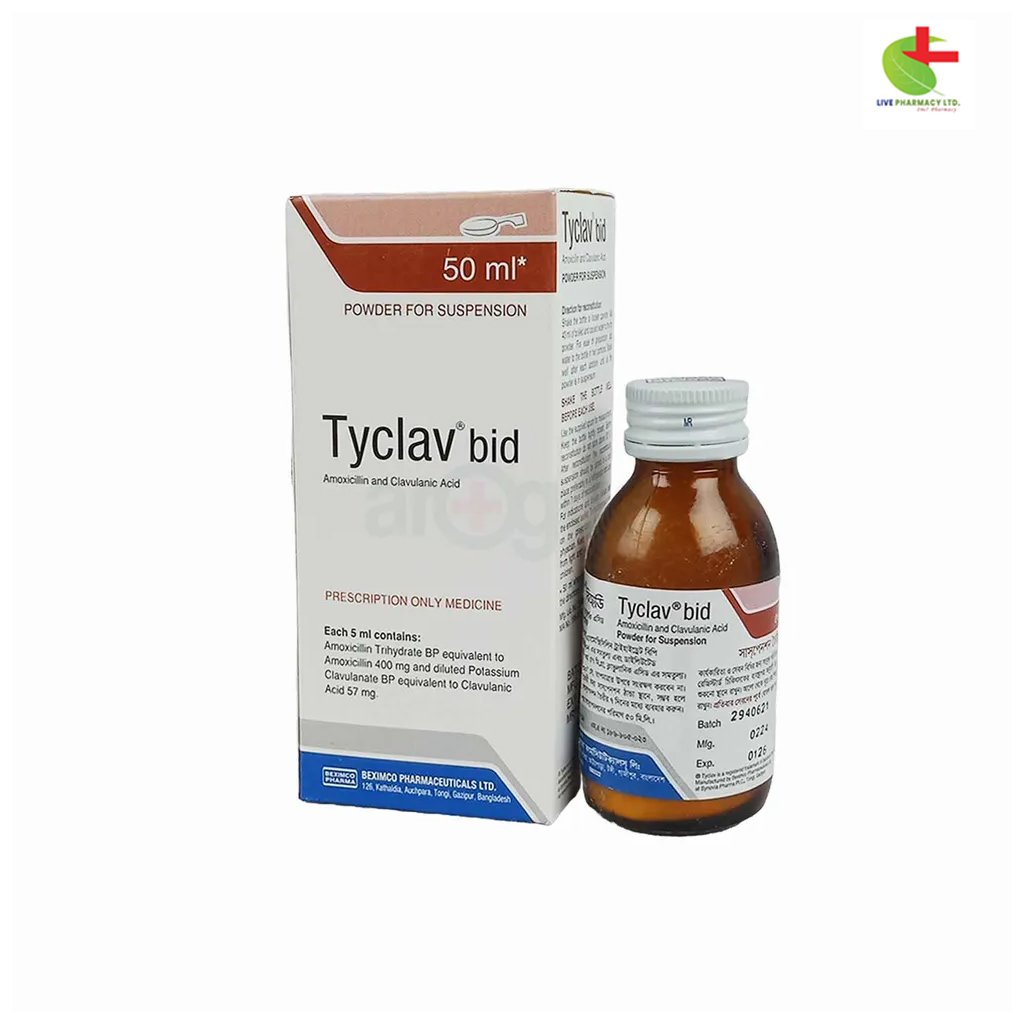
Tyclav BID PFS
230.00৳ Bottle (50 ml)
- Co-amoxiclav is a broad-spectrum antibiotic that combines Amoxicillin and Clavulanic Acid.
- It is effective against various bacterial infections, including respiratory, genito-urinary, skin, bone, and joint infections.
- The combination of Amoxicillin and Clavulanic Acid enhances efficacy by inhibiting beta-lactamase enzymes, thereby extending the antibiotic’s spectrum.
- Available in tablets, suspensions, and injectable forms, dosage depends on the infection’s severity and the patient’s age.
- Always adhere to prescribed dosages and consult a healthcare provider for proper guidance.
 Brand
Brand
|
Beximco Pharmaceuticals Ltd |
|---|---|
 Generics
Generics
|
Amoxicillin + Clavulanic Acid |
 Type
Type
|
Powder for Suspension |
Indications
Co-amoxiclav is prescribed for the short-term management of various bacterial infections, including:
- Upper Respiratory Tract Infections: Such as tonsillitis, sinusitis, and otitis media.
- Lower Respiratory Tract Infections: Including acute and chronic bronchitis, lobar pneumonia, and bronchopneumonia.
- Genito-Urinary Tract Infections: For conditions like cystitis, urethritis, and pyelonephritis.
- Skin and Soft Tissue Infections: Effective for treating various dermatological infections.
- Bone and Joint Infections: Including osteomyelitis.
- Other Infections: Such as septic abortion, puerperal sepsis, and intra-abdominal sepsis.
Composition
Co-amoxiclav is available in multiple forms:
- 375 mg Tablet: Contains 250 mg Amoxicillin (as Amoxicillin Trihydrate BP) and 125 mg Clavulanic Acid (as Diluted Potassium Clavulanate BP).
- 625 mg Tablet: Includes 500 mg Amoxicillin (as Amoxicillin Trihydrate BP) and 125 mg Clavulanic Acid (as Diluted Potassium Clavulanate BP).
- 1 gm Tablet: Provides 875 mg Amoxicillin (as Amoxicillin Trihydrate BP) and 125 mg Clavulanic Acid (as Diluted Potassium Clavulanate BP).
- Powder for Suspension (Standard): Each 5 ml of reconstituted suspension contains 125 mg Amoxicillin (as Amoxicillin Trihydrate BP) and 31.25 mg Clavulanic Acid (as Diluted Potassium Clavulanate BP).
- Powder for Suspension (Forte): Each 5 ml of reconstituted suspension contains 400 mg Amoxicillin (as Amoxicillin Trihydrate BP) and 57.5 mg Clavulanic Acid (as Diluted Potassium Clavulanate BP).
- 1.2 gm Injection: Each vial contains a sterile blend of 1 gm Amoxicillin Sodium BP and 200 mg Clavulanate Potassium USP.
- 0.6 gm Injection: Each vial provides a sterile mixture of 500 mg Amoxicillin Sodium BP and 100 mg Clavulanate Potassium USP.
Pharmacology
Pharmacodynamically, Co-amoxiclav combines Amoxicillin, a broad-spectrum antibiotic, with Clavulanic Acid, a beta-lactamase inhibitor. Amoxicillin is effective against many Gram-positive and Gram-negative microorganisms but can be degraded by beta-lactamases. Clavulanic Acid inactivates these enzymes, protecting Amoxicillin and extending its spectrum of activity. Pharmacokinetically, both components peak in serum about an hour after administration. Optimal absorption occurs when taken at the start of a meal. Both clavulanate and Amoxicillin have low serum binding, with about 70% remaining free in the serum. Doubling the dose roughly doubles serum levels.
Dosage
For Adults and Children Over 12 Years:
- Tablets:
- Standard dosing: One 625 mg Tablet every 12 hours, or one 375 mg Tablet every 8 hours.
- For severe infections or respiratory conditions: One 1 gm Tablet every 12 hours, or one 625 mg Tablet every 8 hours.
- Suspension:
- Children Aged 6-12 Years: 2 teaspoonfuls every 8 hours.
- Children Aged 1-6 Years: 1 teaspoonful every 8 hours.
- Children Under 1 Year: 25 mg/kg/day, divided into doses every 8 hours. For example, a 7.5 kg child would require 2 ml suspension three times daily. Treatment should not exceed 14 days without re-evaluation.
- Forte Suspension:
- Mild to Moderate Infections: 25/3.6 mg/kg/day, suitable for conditions like recurrent tonsillitis, and lower respiratory or skin infections.
- Serious Infections: 45/6.4 mg/kg/day for conditions such as otitis media, bronchopneumonia, and severe urinary tract infections.
- Children Aged 2-12 Years:
- Mild to Moderate Infections:
- 25/3.6 mg/kg/day:
- Ages 2-6 years (13-21 kg): 2.5 ml suspension twice daily.
- Ages 7-12 years (22-40 kg): 5 ml suspension twice daily.
- 25/3.6 mg/kg/day:
- Serious Infections:
- 45/6.4 mg/kg/day:
- Ages 2-6 years (13-21 kg): 5 ml Forte suspension twice daily.
- Ages 7-12 years (22-40 kg): 10 ml Forte suspension twice daily.
- 45/6.4 mg/kg/day:
- Mild to Moderate Infections:
- IV Injection:
- Adults:
- Typically, 1.2 gm every 8 hours. For more severe cases, increase to 1.2 gm every 6 hours.
- For surgical prophylaxis: 1.2 gm at induction; in high-risk procedures (e.g., colorectal surgery), doses up to 2-3 gm every 8 hours may be used.
- Children:
- 0 to 3 Months: 30 mg/kg every 8 hours (or every 12 hours in perinatal and premature infants).
- 3 Months to 12 Years: Typically, 30 mg/kg every 8 hours, increasing to every 6 hours for severe infections.
- Adults:
Administration
Oral dosages can be taken with or without food, but absorption is enhanced when taken at the start of a meal. For IV injection, it is administered intravenously by injection or slow infusion. The reconstituted solution must be used within 20 minutes.
Interaction
Co-amoxiclav may prolong bleeding time and prothrombin time. It can reduce the efficacy of oral contraceptives and may interact with allopurinol, increasing the risk of allergic skin reactions. There are no specific data on the concurrent use of Co-amoxiclav and allopurinol.
Contraindications
Co-amoxiclav is contraindicated in patients with hypersensitivity to penicillins or other beta-lactam antibiotics and those with a history of cholestatic jaundice from Co-amoxiclav or Penicillin.
Side Effects
Side effects are typically mild and transient, including diarrhea, nausea, and rash. Rarely, more serious conditions such as Stevens-Johnson Syndrome or exfoliative dermatitis may occur. Gastrointestinal issues may be minimized by taking the medication with meals.
Pregnancy & Lactation
Co-amoxiclav has shown no teratogenic effects in animal studies. Use during pregnancy is not recommended unless essential. Trace amounts of Amoxicillin may be present in breast milk.
Precautions & Warnings
Use Co-amoxiclav with caution in patients on anticoagulant therapy or with severe hepatic dysfunction. Dosage adjustments are necessary for renal impairment. High doses should be administered with adequate fluid intake to prevent crystalluria.
Use in Special Populations
Dosage adjustments are required for patients with renal impairment. For children, similar dosage reductions apply. Hepatic impairment requires cautious dosing and regular monitoring.
Overdose Effects
Overdose may result in gastrointestinal symptoms and electrolyte imbalances. Hemodialysis can help remove the drug from circulation.
Therapeutic Class
Broad-spectrum penicillins.
Reconstitution
For IV injection, reconstitute 1.2 gm with 20 ml Water for Injection BP. Avoid mixing with dextrose, bicarbonate solutions, or proteinaceous fluids. The reconstituted solution can be injected into infusion fluids containing glucose, bicarbonate, and dextran.
Storage Conditions
Store below 25°C, protected from light and moisture. Refrigerate reconstituted suspension and use within 7 days. Use reconstituted vials within 20 minutes.











Reviews
There are no reviews yet.