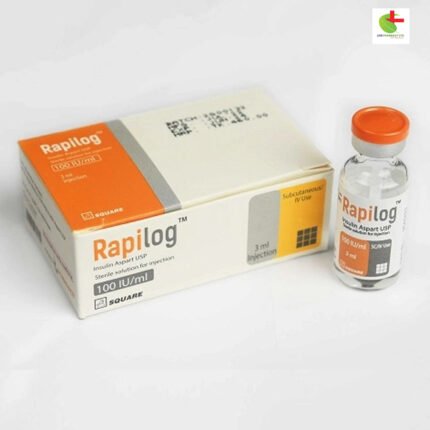
Rapilog Cartridge
560.00৳ Cartridge
- Explore Rapilog, a rapid-acting human insulin analog at Live Pharmacy.
- Designed to enhance glycemic control in adults and children with diabetes mellitus.
- Rapilog offers an earlier onset and intermediate duration of action.
- Regulates glucose metabolism effectively, aiding in comprehensive diabetes management.
- Trust Live Pharmacy for expert guidance and quality medications tailored to your needs.
 Brand
Brand
|
Square Pharmaceuticals PLC |
|---|---|
 Generics
Generics
|
Insulin Aspart |
Indications
Rapilog, a rapid-acting human insulin analog, is indicated for enhancing glycemic control in adults and children with diabetes mellitus.
Composition
Each ml suspension of Rapilog contains 100 IU (equivalent to 3.50 mg) of Insulin Aspart (rDNA) BP, comprising 30% soluble Insulin Aspart and 70% protamine-crystallized Insulin Aspart.
Description
Rapilog is a sterile suspension of human insulin analog, consisting of 30% soluble Rapilog and 70% protamine-crystallized Rapilog. It acts as a blood glucose-lowering agent with an earlier onset and intermediate duration of action.
Pharmacology
Insulin Aspart primarily regulates glucose metabolism by binding to insulin receptors on muscle and fat cells. This facilitates the cellular uptake of glucose while inhibiting glucose output from the liver.
Dosage
The dosage of Insulin Aspart is individualized by the physician based on patient needs. It can be used in mono-therapy or in combination with oral antidiabetic drugs for type 2 diabetes management. Specific dosage recommendations are provided for both type 1 and type 2 diabetes patients.
Administration
Rapilog should be administered 5-10 minutes before meals.
Interaction
Several drugs may affect glucose metabolism and require dose adjustments when used concomitantly with Rapilog. These interactions should be carefully monitored and managed under medical supervision.
Contraindications
Rapilog is contraindicated during episodes of hypoglycemia and in patients with hypersensitivity to Insulin Aspart or its excipients.
Side Effects
Common side effects of Rapilog include hypoglycemia, allergic reactions, injection site reactions, lipodystrophy, pruritus, and rash.
Pregnancy & Lactation
Insulin Aspart is classified as pregnancy category B, with no restrictions on its use during lactation. However, dosage adjustments may be necessary.
Precautions & Warnings
Blood glucose levels should be monitored regularly in all patients treated with insulin. Rapilog regimens should be modified cautiously and under medical supervision.
Use in Special Populations
Special considerations may be required for patients with renal or hepatic impairment, necessitating potential dose adjustments.
Overdose Effects
Although a specific overdose for insulin cannot be defined, hypoglycemia may develop with excessively high doses. Treatment of hypoglycemic episodes should be administered promptly and in accordance with medical guidelines.
Therapeutic Class
Rapilog belongs to the class of rapid-acting insulin.
Storage Conditions
Rapilog should be stored in a refrigerator at 2°C to 8°C, protected from light, and should not be frozen.







Reviews
There are no reviews yet.