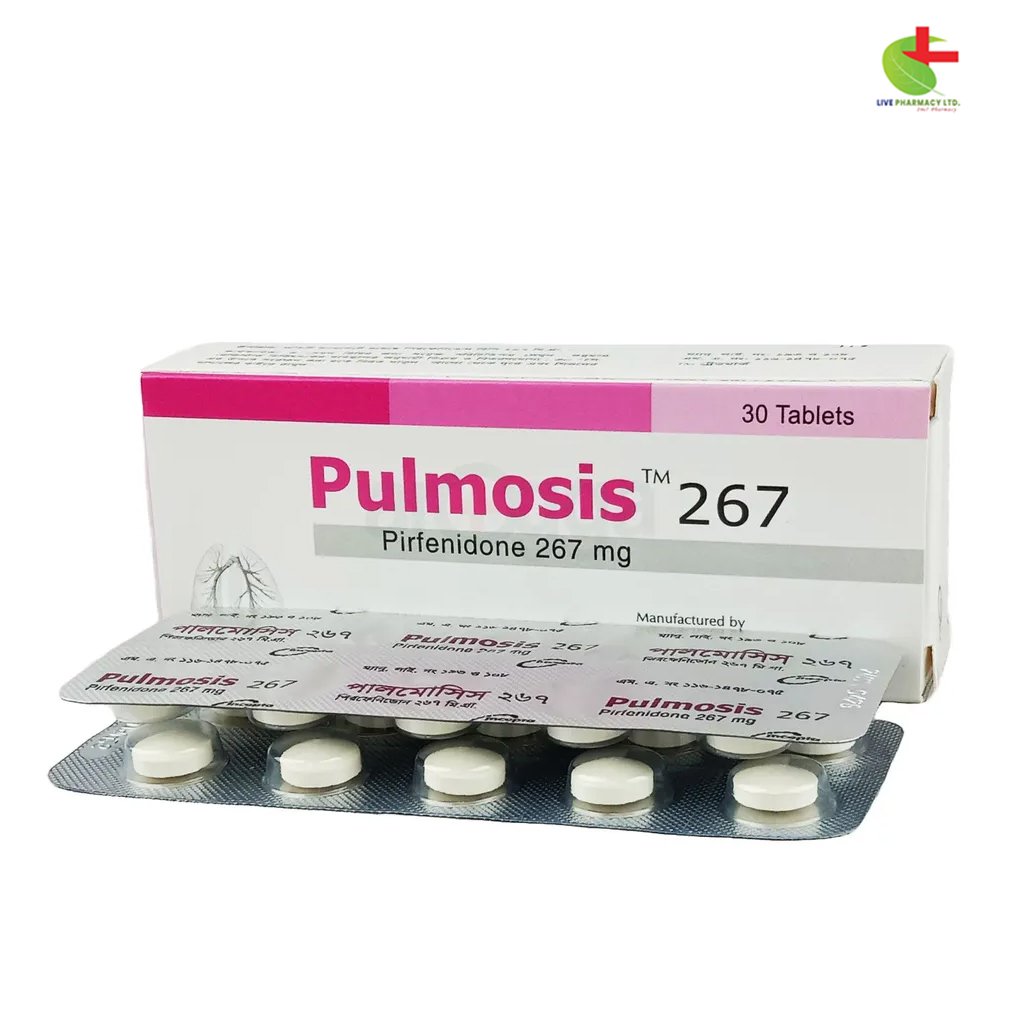
Pulmosis 267
450.00৳ Strip
- Pulmosis is an innovative treatment for mild to moderate Idiopathic Pulmonary Fibrosis (IPF) in adults, utilizing the active ingredient pirfenidone.
- This medication possesses anti-inflammatory, antioxidant, and anti-fibrotic properties, potentially improving lung function and reducing acute exacerbations.
- Recommended for oral administration with food, Pulmosis helps manage the progression of IPF while requiring careful monitoring for side effects.
- Always consult a healthcare professional before use.
 Brand
Brand
|
Incepta Pharmaceuticals Ltd |
|---|---|
 Generics
Generics
|
Pirfenidone |
 Type
Type
|
Capsule |
Indications
Pulmosis is recommended for adults to treat mild to moderate Idiopathic Pulmonary Fibrosis (IPF).
Consult a registered healthcare professional before use.
Pharmacology
Pirfenidone is an innovative medication known for its anti-inflammatory, antioxidant, and anti-fibrotic properties. It may enhance lung function and decrease the frequency of acute exacerbations in patients with IPF. While the exact mechanism of action is still being studied, one key anti-fibrotic effect involves the suppression of TGF-β1 (transforming growth factor-β1), a crucial cytokine in fibrogenesis and extracellular matrix formation. Evidence also suggests that pirfenidone can reduce levels of pro-inflammatory cytokines such as TNF-α, interleukin-1, and interferon-gamma. In animal studies, it has shown the ability to inhibit inflammatory cell influx and decrease pulmonary vascular permeability induced by bleomycin.
Dosage & Administration
- Route: Administered orally with food.
Adults: Gradually increase to the recommended daily dose of nine 267 mg tablets or three 801 mg tablets over a 14-day period as follows:
- Days 1 to 7: One 267 mg tablet three times daily (total 801 mg/day)
- Days 8 to 14: Two 267 mg tablets three times daily (total 1602 mg/day)
- Day 15 onward: Three 267 mg tablets or one 801 mg tablet three times daily (total 2403 mg/day)
The recommended daily dose is three 267 mg tablets or one 801 mg tablet taken three times a day with food for a total of 2403 mg/day. Doses exceeding 2403 mg/day are not advisable. If a patient misses 14 consecutive days or more, they should restart the initial 2-week titration regimen. For interruptions of less than 14 days, resume the previous recommended daily dose without titration.
Dose Adjustments and Considerations:
- Gastrointestinal events: If gastrointestinal side effects occur, take the medication with food. If symptoms persist, reduce the dose to 1-2 tablets (267 mg-534 mg) 2-3 times daily with food and gradually re-escalate as tolerated. If symptoms continue, consider a 1-2 week treatment interruption.
- Photosensitivity or rash: Advise patients to use sunblock and avoid sun exposure. Reduce the dose to three 267 mg tablets/day (one 267 mg tablet three times a day). If rash persists after 7 days, discontinue Pirfenidone for 15 days, then re-escalate as needed. Severe reactions warrant immediate medical advice.
- Hepatic function: In case of significant ALT/AST elevation, adjust the dose or discontinue treatment as per guidelines.
Consult a registered healthcare professional before use.
Interactions
- With medicine: Moderate (e.g., ciprofloxacin) and strong inhibitors of CYP1A2 (e.g., fluvoxamine) may increase systemic exposure to Pulmosis and alter its adverse effects. Discontinue fluvoxamine prior to Pulmosis or reduce to 267 mg three times a day. Consider reducing dosage with ciprofloxacin.
- With food: No interactions noted. A lower incidence of adverse reactions was observed in patients taking the medication with food compared to fasting.
Contraindications
- Hypersensitivity to pirfenidone or any excipients
- Concomitant use of fluvoxamine
- Severe hepatic impairment or end-stage liver disease
- Severe renal impairment (CrCl <30 ml/min) or end-stage renal disease requiring dialysis
Side Effects
- Common: Nausea, rash, abdominal pain, upper respiratory infections, diarrhea, fatigue, headache, dyspepsia, dizziness, vomiting, anorexia, gastroesophageal reflux disease, sinusitis, insomnia, weight loss, arthralgia.
- Rare: Photosensitivity, decreased appetite, pruritus, asthenia, dysgeusia, non-cardiac chest pain.
Pregnancy & Lactation
- Pregnancy: Limited data available. Animal studies show potential placental transfer and adverse effects on gestation and fetal viability at high doses. It’s advisable to avoid Pirfenidone during pregnancy.
- Lactation: Uncertain if pirfenidone is excreted in human milk. Animal studies indicate it may accumulate in milk. Consider the risks of breast-feeding versus the benefits of treatment.
- Fertility: No adverse effects on fertility were noted in rats at doses up to 1000 mg/kg/day.
Precautions & Warnings
- Elevated liver enzymes: Monitor ALT, AST, and bilirubin levels before and during treatment. Adjust or discontinue as needed.
- Photosensitivity and rash: Avoid sun exposure. Use sunscreen and protective clothing.
- Gastrointestinal disorders: Nausea, vomiting, and abdominal pain may require dose adjustments or treatment interruption.
Use in Special Populations
- Children & Adolescents: Safety and efficacy in this group are not established.
- Hepatic Impairment: Monitor for adverse reactions; dosage modifications may be necessary.
- Renal Impairment: Similar monitoring and possible dosage adjustments are required.
- Smokers: Decreased drug exposure may affect efficacy.
Overdose Effects
Limited clinical experience with overdose exists. Doses of up to 4806 mg/day have been administered with mild and transient adverse reactions consistent with known side effects.
Therapeutic Class
Immunosuppressant
Storage Conditions
Store in a cool, dry place, away from light and moisture. Keep out of reach of children.










Reviews
There are no reviews yet.