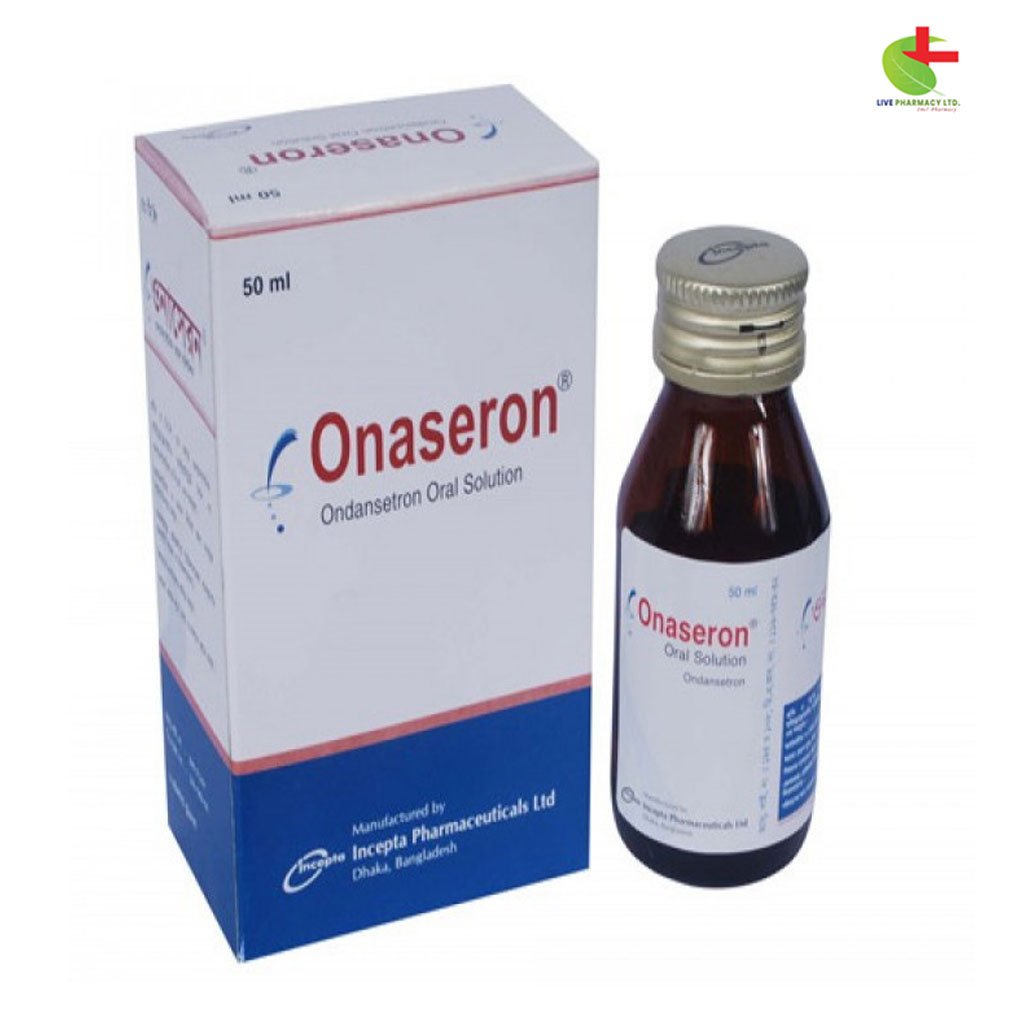
Onaseron
45.00৳ Bottle (50ml)
- Onaseron is a serotonin 5-HT3 receptor antagonist used for preventing nausea and vomiting caused by chemotherapy, radiotherapy, and post-surgery.
- It is available in oral soluble film, tablets, and injections, offering convenient administration options.
- Onaseron works by blocking the vomiting reflex triggered by chemotherapy and radiotherapy.
- Common side effects include headache, constipation, and mild rash; severe reactions are rare.
 Brand
Brand
|
Incepta Pharmaceuticals Ltd |
|---|---|
 Generics
Generics
|
Ondansetron |
 Type
Type
|
Oral Solution |
Indications for Onaseron
Onaseron is a potent 5-HT3 receptor antagonist, effective for the prevention and treatment of nausea and vomiting in the following conditions:
- Prevention of nausea and vomiting due to emetogenic cancer chemotherapy (both initial and repeat courses).
- Prevention and treatment of post-operative nausea and vomiting.
- Prevention of nausea and vomiting induced by radiotherapy.
Note: Use Onaseron as directed by a registered healthcare professional.
Product Description
Onaseron is available as an oral soluble film, designed to dissolve rapidly on the tongue within 20 seconds, without the need for water. The film can be easily swallowed after it dissolves, offering a convenient, water-free option for nausea and vomiting relief. The active ingredient, Ondansetron, is a selective serotonin 5-HT3 receptor antagonist, which helps to block the receptors responsible for initiating nausea and vomiting. The molecular formula for Ondansetron is C18H19N3O, with a molecular weight of 293.3.
Pharmacological Action
Ondansetron is a highly selective 5-HT3 receptor antagonist that works to prevent nausea and vomiting caused by chemotherapy, radiotherapy, and surgery. Though its exact mechanism is not fully understood, it is believed that chemotherapy and radiotherapy trigger the release of serotonin (5-HT) in the intestines, which activates the vomiting reflex. Ondansetron works by blocking these 5-HT3 receptors, both in the peripheral and central nervous systems, to prevent this reflex. For post-operative nausea and vomiting (PONV), the mechanism of action is presumed to be similar to that for chemotherapy-induced nausea.
Dosage Guidelines
Chemotherapy-Induced Nausea and Vomiting:
- Adults and Pediatric Patients (6 months to 18 years):
- 8 mg orodispersible tablet: Administer 0.15 mg/kg in three doses, up to 16 mg per dose.
- Injection: Administer 0.15 mg/kg in three doses, up to 16 mg per dose, via intravenous infusion over 15 minutes.
Radiotherapy-Induced Nausea and Vomiting:
- Adults:
- Initial dose: 8 mg orally, 1-2 hours before radiotherapy.
- Post-treatment: 8 mg orally every 8 hours for up to 5 days post-treatment.
Postoperative Nausea and Vomiting:
- Adults:
- 16 mg total (administered as two 8 mg tablets).
- Pediatrics (>40 kg): 4 mg injection.
- Pediatrics (40 kg or under): 0.1 mg/kg injection.
Administration of Oral Soluble Film
- Tear open the pouch carefully.
- Place the Onaseron film on top of your tongue, where it will dissolve within 20 seconds.
- Do not chew or swallow the film whole.
- Once dissolved, swallow the film with or without liquid.
- Wash hands after use.
Drug Interactions
Onaseron does not significantly influence the cytochrome P-450 drug-metabolizing enzymes. However, drugs that affect these enzymes may alter the metabolism of Onaseron. No dosage adjustments are needed for patients taking such drugs based on available data.
Contraindications
Onaseron is contraindicated in patients with known hypersensitivity to Ondansetron or any of its ingredients, and should not be used in conjunction with apomorphine.
Side Effects
Common side effects include headache, constipation, and diarrhea, generally mild to moderate. In some cases, rash, flushing, and liver enzyme abnormalities may occur. Rarely, severe allergic reactions, bronchospasm, and tachycardia have been reported. However, the relationship to Onaseron is unclear.
Pregnancy & Lactation
Although no carcinogenic effects were noted in animal studies, there are insufficient data from human studies to confirm safety during pregnancy. Ondansetron is excreted in the breast milk of rats, so it is recommended to exercise caution when administering to nursing mothers.
Precautions & Warnings
Onaseron should not replace nasogastric suction in post-surgical or chemotherapy patients. It is also important to monitor for signs of ileus or gastric distension in such patients.
Use in Special Populations
- Renal Impairment: No dosage adjustment is needed.
- Hepatic Impairment: In severe cases, a single dose of 8 mg should be infused over 15 minutes prior to chemotherapy.
- Pediatrics (under 4 years): Limited information available; use with caution.
- Elderly: No dosage adjustment needed.
Therapeutic Class
Anti-emetic drugs.
Storage Conditions
Store at room temperature (not exceeding 30ºC) in a dry place. Protect from light and moisture.







Reviews
There are no reviews yet.