
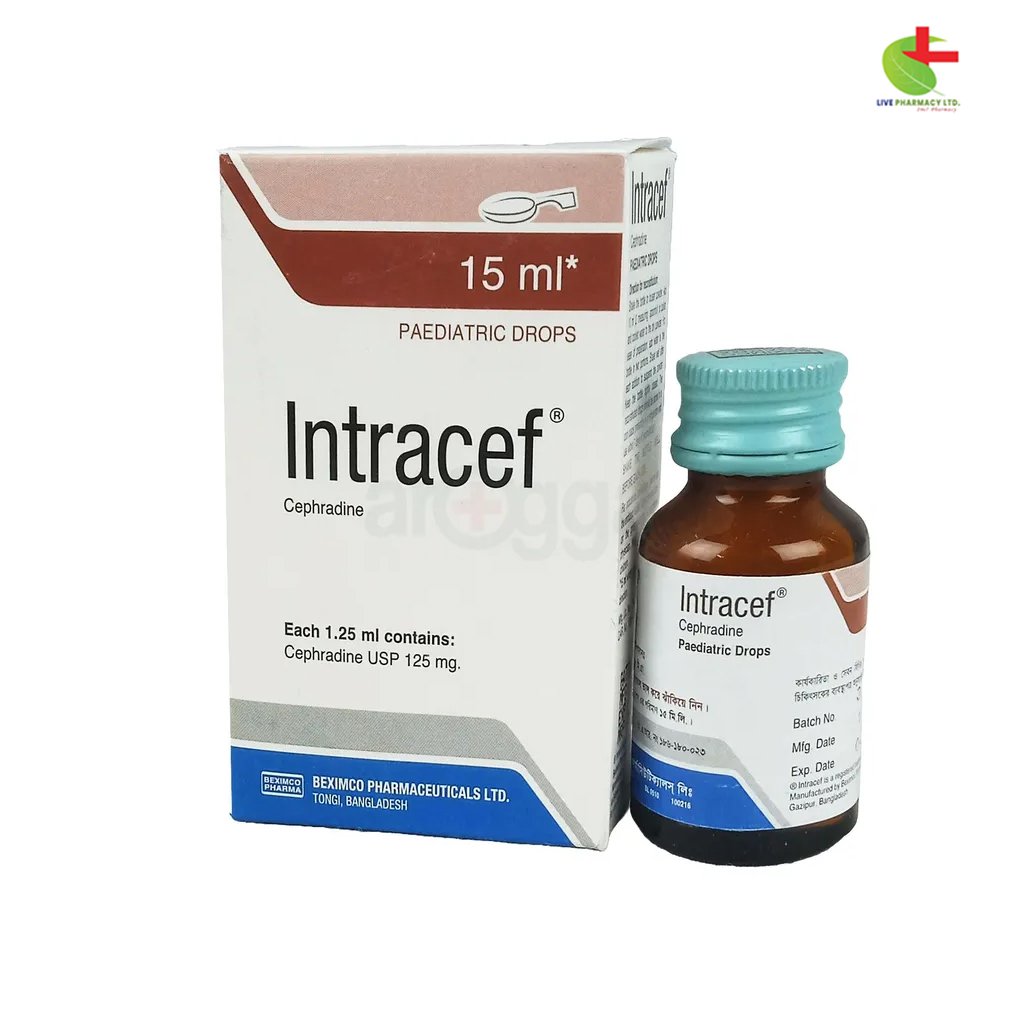
Intracef Pediatric Drops
50.00৳ Bottle (15 ml)
- Intracef is a broad-spectrum cephalosporin antibiotic.
- Effective against Gram-positive and Gram-negative bacteria.
- Used for treating upper and lower respiratory tract infections, urinary tract infections, and skin infections.
- Provides strong bactericidal activity and remains stable against penicillinase-producing strains.
 Brand
Brand
|
Beximco Pharmaceuticals Ltd |
|---|---|
 Generics
Generics
|
Cephradine |
 Type
Type
|
Paediatric Drop |
Indications
Intracef, an oral cephalosporin antibiotic, is designed to treat infections caused by both Gram-positive and Gram-negative bacteria. It is effective for:
- Upper Respiratory Tract Infections (URTIs): Includes sinusitis, pharyngitis, tonsillitis, laryngotracheobronchitis, and otitis media.
- Lower Respiratory Tract Infections (LRTIs): Such as acute and chronic bronchitis, lobar pneumonia, and bronchopneumonia.
- Urinary Tract Infections (UTIs): Treats cystitis, urethritis, and pyelonephritis.
- Skin and Soft Tissue Infections: Addresses conditions like abscesses, cellulitis, furunculosis, and impetigo.
Intracef is effective against a range of pathogens, including:
- Gram-positive bacteria: Staphylococci (including penicillin-resistant and penicillinase-producing strains), Streptococci, Streptococcus pyogenes, and Streptococcus pneumoniae.
- Gram-negative bacteria: Escherichia coli, Klebsiella spp., Proteus mirabilis, Haemophilus influenzae, Shigella spp., Salmonella spp. (including Salmonella typhi), and Neisseria spp.
It remains effective even against strains producing penicillinase, which usually confer resistance to ampicillin.
Pharmacology
Intracef (cephradine) is a semi-synthetic, broad-spectrum cephalosporin antibiotic. It acts by disrupting the bacterial cell wall synthesis, leading to bacterial cell lysis. Cephradine is effective against both penicillinase-producing and non-producing staphylococci. It achieves high plasma concentrations and maintains stability against many beta-lactamase enzymes.
Dosage & Administration
Oral Administration:
- Adults:
- UTIs: 500 mg four times daily or 1 g twice daily; higher doses may be needed for severe cases.
- Respiratory Tract Infections: 250-500 mg four times daily or 500 mg-1 g twice daily, depending on severity.
- Skin and Soft Tissue Infections: 250-500 mg four times daily or 500 mg-1 g twice daily, depending on infection severity.
- Children:
- General Dose: 25-50 mg/kg daily in two or four divided doses.
- Otitis Media: 75-100 mg/kg daily in divided doses every 6 to 12 hours.
- Maximum Daily Dose: 4 g.
- Elderly: Normal adult dosing applies, but renal and hepatic function should be monitored.
Injectable Administration:
- Adults: 2-4 g daily in four divided doses, up to 8 g daily. For prophylaxis, a single dose of 1-2 g intramuscularly or intravenously is recommended.
- Children: 50-100 mg/kg daily in four divided doses, up to 300 mg/kg daily for severe infections.
Interactions
Caution is advised when using Intracef with nephrotoxic drugs like aminoglycosides, diuretics (e.g., frusemide), and probenecid due to increased risk of renal toxicity.
Contraindications
Intracef should not be used by individuals with known hypersensitivity to cephalosporins.
Side Effects
Intracef is generally well-tolerated. Possible side effects include gastrointestinal disturbances (e.g., nausea, diarrhea), hypersensitivity reactions (e.g., rash, urticaria), and rare cases of more severe effects like blood disorders or liver issues. Consult a healthcare provider if severe reactions occur.
Pregnancy & Lactation
Safety in pregnancy has not been established, although animal studies show no teratogenic effects. Cephradine is excreted in breast milk; use with caution in lactating mothers. Patients should avoid operating heavy machinery if experiencing dizziness.
Precautions & Warnings
- Prolonged use may lead to superinfection due to resistant organisms.
- Caution is advised for those with a history of penicillin sensitivity due to potential cross-reactivity.
- Intracef may cause false-positive urine glucose results with certain tests.
- Renal impairment requires dosage adjustment.
- Contains lactose; not suitable for those with galactose intolerance or related conditions.
Use in Special Populations
- Renal Impairment: Adjust doses based on creatinine clearance.
- Haemodialysis: Special dosing guidelines are provided.
Overdose Effects
Overdose symptoms include nausea, vomiting, and diarrhea. Treatment is primarily supportive, with gastric lavage considered for significant ingestion.
Therapeutic Class
First-generation Cephalosporins.
Storage Conditions
Prepare Intracef Suspension fresh. Use reconstituted suspension within 7 days at room temperature or 14 days refrigerated. Injectable solutions should be used within 2 hours at room temperature or 12 hours refrigerated. Store below 30°C, away from light and moisture. Keep out of reach of children. Dispense only with a physician’s prescription.




Reviews
There are no reviews yet.