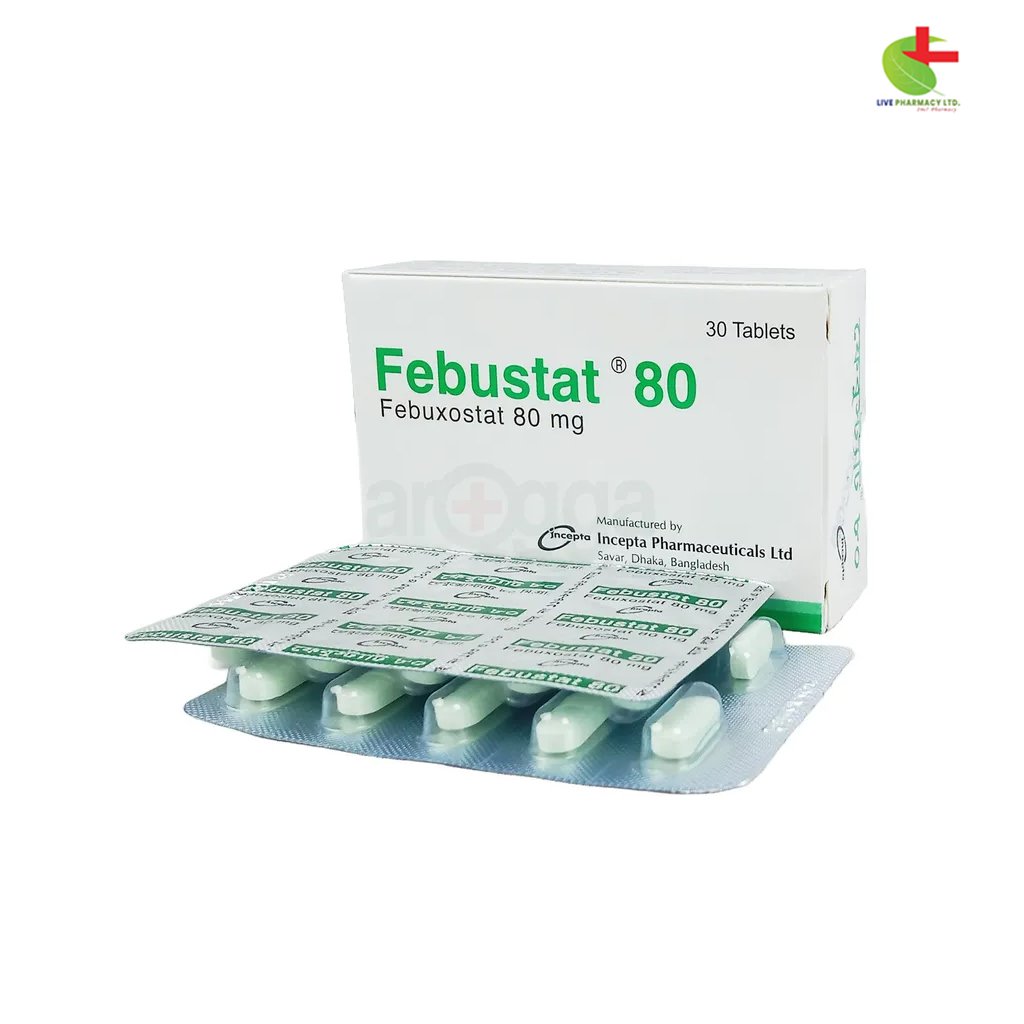
Febustat 80
220.00৳ Strip
- Febuxostat tablets effectively treat chronic hyperuricemia in gout patients, targeting uric acid reduction.
- As a selective xanthine oxidase inhibitor, it helps manage gout flares with minimal side effects.
- Not recommended for asymptomatic hyperuricemia; use under a registered physician’s supervision.
- Regular monitoring is advised to ensure optimal treatment outcomes.
 Brand
Brand
|
Incepta Pharmaceuticals Ltd |
|---|---|
 Generics
Generics
|
Febuxostat |
 Type
Type
|
Tablet |
Indications
Febuxostat tablets are prescribed for the long-term management of hyperuricemia in patients diagnosed with gout. They are not suitable for treating asymptomatic hyperuricemia.
Use only under the guidance of a registered physician.
Pharmacology
Febuxostat is a selective xanthine oxidase (XO) inhibitor that is non-purine based. It effectively lowers serum uric acid levels by inhibiting the activity of xanthine oxidase, the enzyme responsible for uric acid production. Xanthine oxidase catalyzes the conversion of hypoxanthine to xanthine and subsequently to uric acid. At therapeutic levels, Febuxostat is not expected to inhibit other enzymes involved in the synthesis and metabolism of purines and pyrimidines.
Dosage & Administration
- Initial Dose: Start with one 40 mg Febuxostat tablet taken daily.
- If after two weeks the serum uric acid level remains above 6 mg/dL, increase the dose to one 80 mg tablet daily.
- For patients still not achieving target levels after 2-4 weeks on the 80 mg dose, the dosage can be raised to one 120 mg tablet daily.
For Tumor Lysis Syndrome:
Administer one 120 mg Febuxostat tablet daily, beginning two days prior to cytotoxic therapy and continuing for at least 7 days. Treatment may extend to 9 days depending on the chemotherapy duration, based on clinical judgment.
Gout Flares:
Patients may experience gout flares when initiating Febuxostat treatment due to fluctuations in serum uric acid levels that mobilize urate from tissue deposits. It is advisable to initiate prophylaxis with a non-steroidal anti-inflammatory drug (NSAID) or colchicine. Should a gout flare occur, it is unnecessary to discontinue Febuxostat; instead, manage the flare according to the patient’s needs.
Renal and Hepatic Impairment:
No dose adjustment is required for patients with mild to moderate renal or hepatic impairment.
Pediatric Use:
The safety and efficacy of Febuxostat in individuals under 18 years have not been established.
Use only under the guidance of a registered physician.
Interactions
- Mercaptopurine/Azathioprine: Concurrent use is not recommended due to potential toxicity from increased plasma concentrations.
- Rosiglitazone/CYP2C8 Substrates: No dose adjustment is required for these medications when used with Febuxostat.
- Naproxen and Other Inhibitors of Glucuronidation: Co-administration is safe without dose adjustments.
- Inducers of Glucuronidation: Monitor serum uric acid levels after starting treatment with potent inducers, as they may affect Febuxostat’s efficacy.
- Colchicine/Indometacin/Hydrochlorothiazide/Warfarin: Febuxostat can be safely co-administered without dose adjustments.
Antacids:
Taking antacids containing magnesium hydroxide and aluminum hydroxide may delay Febuxostat absorption by approximately one hour but does not significantly affect overall drug levels. Thus, Febuxostat can be taken without concern for antacid use.
Contraindications
Febuxostat is contraindicated in patients taking azathioprine, mercaptopurine, or theophylline.
Side Effects
Common side effects include gout flares, liver function abnormalities, diarrhea, nausea, headache, rash, and edema, typically mild to moderate. Rare but serious hypersensitivity reactions have been reported.
Pregnancy & Lactation
Classified as Pregnancy Category C. There are insufficient studies in pregnant women, and Febuxostat should only be used if the potential benefits justify the risks to the fetus. It is unclear if the drug is excreted in human milk, so caution is advised when administering to nursing mothers.
Precautions & Warnings
- Gout Flare: Increased flares can occur at treatment initiation. If a flare arises, do not discontinue Febuxostat; consider prophylactic therapy for up to six months.
- Cardiovascular Events: Clinical trials have noted a higher incidence of cardiovascular thromboembolic events compared to allopurinol. Monitor for symptoms of myocardial infarction and stroke.
- Liver Enzyme Elevation: Regular monitoring of liver function tests is advised, as elevations in transaminases have been observed.
Overdose Effects
Febuxostat has been safely administered at doses up to 300 mg daily for seven days in healthy subjects without significant toxicity.
Therapeutic Class
Medications for Gout
Storage Conditions
Store below 30°C, away from light and moisture. Keep out of reach of children.





Reviews
There are no reviews yet.