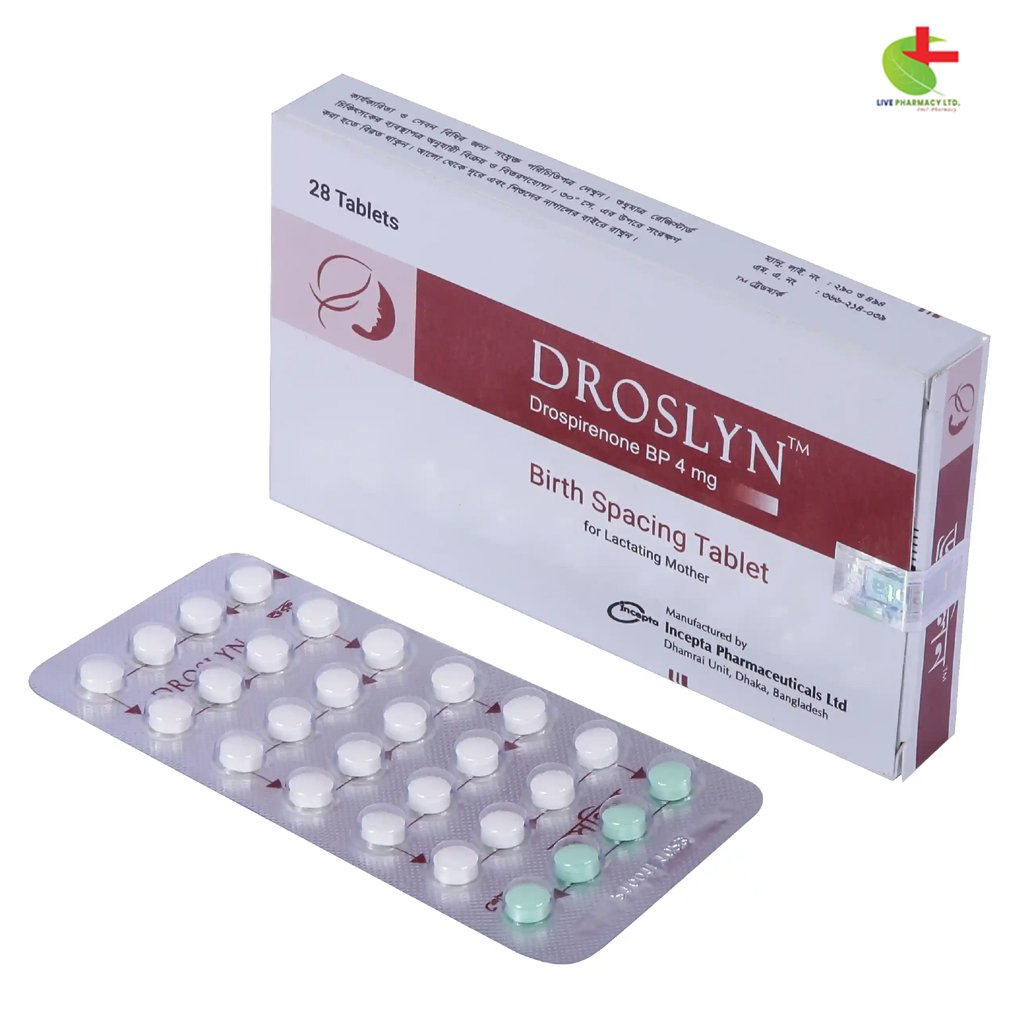
Droslyn
400.00৳ Strip
- Droslyn is a progestin-based oral contraceptive designed to prevent pregnancy by suppressing ovulation.
- It includes Drospirenone, a spironolactone analogue, offering additional benefits like anti-mineralocorticoid activity.
- Taken daily for 28 days, Droslyn helps regulate menstrual cycles and reduces the risk of pregnancy when used correctly.
- Always consult a healthcare provider before use to ensure it’s the right choice for you.
 Brand
Brand
|
Incepta Pharmaceuticals Ltd |
|---|---|
 Generics
Generics
|
Drospirenone |
 Type
Type
|
Tablet |
Indications
Droslyn is a progestin-based oral contraceptive designed for females to prevent pregnancy.
Consult with a registered healthcare provider before using this medication.
Pharmacology
Drospirenone, the active ingredient in Droslyn, is a progestin used to prevent pregnancy in females with reproductive potential. This oral contraceptive works primarily by suppressing ovulation. Drospirenone is a spironolactone analogue and also exhibits anti-mineralocorticoid activity, which contributes to its contraceptive effectiveness.
- Absorption: Drospirenone’s absorption is dose-proportional for single doses between 1-10 mg. After a single dose, peak plasma concentrations (Cmax) of approximately 27 ng/ml are achieved within 2-6 hours. During continuous use, steady-state serum concentrations of about 41 ng/ml are reached after 10 days of treatment. Food does not affect the extent of absorption.
- Distribution: Drospirenone is 95-97% bound to serum albumin and does not bind to sex hormone-binding globulin (SHBG) or corticosteroid-binding globulin (CBG). Its apparent volume of distribution is approximately 4 L/kg.
- Metabolism: Drospirenone undergoes extensive metabolism. Its primary metabolites, including the acid form generated by opening the lactone ring and the 4,5-dihydro Drospirenone-3-sulfate, are pharmacologically inactive. Drospirenone is metabolized by the enzyme CYP3A4.
- Excretion: Drospirenone has a terminal half-life of around 30 hours. It is excreted predominantly through feces, with only trace amounts appearing in urine. Metabolism of Drospirenone is extensive, and unchanged Drospirenone is minimally excreted.
Dosage & Administration
Droslyn is taken orally as one tablet per day for 28 consecutive days. The regimen consists of 24 days of white active tablets followed by 4 days of light pink inert tablets. It’s important to take the tablets at the same time each day to maintain a consistent 24-hour interval between doses.
Starting Droslyn for the First Time:
- Day 1 Start: Begin by taking the first white active tablet on the first day of your menstrual cycle.
- Take one white active tablet daily for 24 days.
- After finishing the active tablets, take one light pink inert tablet daily for the next 4 days.
- Start each new pack on the same day of the week as the previous cycle (after finishing the inert tablets).
Switching to Droslyn from Another Contraceptive Method:
- If switching from a combined oral contraceptive (COC), start Droslyn on the day the next pack of your previous contraceptive would have been used.
- For transdermal patches, vaginal rings, injections, intrauterine contraceptives, or implants, start Droslyn on the day you would have scheduled the next application, insertion, injection, or removal.
Refer to the Patient Information for additional guidance on proper use.
Always follow your healthcare provider’s instructions regarding medication use.
Drug Interactions
- Drugs Decreasing the Effectiveness of Droslyn: Enzyme inducers (e.g., efavirenz, phenytoin, rifampicin, St. John’s Wort) may lower the systemic levels of Droslyn and decrease its contraceptive efficacy. Patients using these medications should consider non-hormonal backup contraception during and for 28 days after discontinuing these drugs.
- Drugs Increasing the Effectiveness of Droslyn: When Droslyn is used with strong CYP3A4 inhibitors like ketoconazole, its systemic exposure may increase moderately.
- Potential for Increased Potassium Levels: Droslyn may raise serum potassium levels, particularly in females using medications such as ACE inhibitors, potassium-sparing diuretics, or NSAIDs. Monitoring potassium levels is recommended, especially in those at risk for hyperkalemia.
Contraindications
Droslyn should not be used by females with:
- Renal impairment
- Adrenal insufficiency
- Progestin-sensitive cancers
- Liver tumors or hepatic disorders
- Undiagnosed abnormal uterine bleeding
Side Effects
Common side effects may include:
- Hyperkalemia
- Bleeding irregularities (e.g., breakthrough bleeding) and amenorrhea
- Acne
- Breast pain and tenderness
- Weight gain
- Nausea
- Decreased libido
- Dysmenorrhea (painful periods)
As with all medications, side effects vary between individuals. Please consult your healthcare provider if you experience any adverse reactions.
Pregnancy & Lactation
There is no significant risk of birth defects associated with accidental use of progestins like Drospirenone during early pregnancy. However, it is advised to discontinue Droslyn if pregnancy occurs. Drospirenone is excreted in breast milk in minimal amounts and is unlikely to affect breastfed infants at therapeutic doses.
Precautions & Warnings
- Hyperkalemia: Due to its anti-mineralocorticoid effects, Droslyn may increase potassium levels. Monitor females at risk of hyperkalemia, particularly those taking other potassium-elevating medications.
- Thromboembolic Disorders: Although studies suggest progestin-only methods like Droslyn have a lower risk of thromboembolism than combined oral contraceptives, caution is needed in women with a history of thromboembolic events or those in the postpartum period.
- Liver Disease: Discontinue Droslyn if liver issues such as jaundice develop, and only resume use once liver function returns to normal.
- Bone Density: Long-term use of Droslyn may reduce estradiol levels, potentially affecting bone mineral density, though this risk is not yet fully established.
- Ectopic Pregnancy: Women who experience lower abdominal pain while using Droslyn should be evaluated for ectopic pregnancy.
- Hyperglycemia in Diabetic Patients: Some females with diabetes may experience a reduction in insulin sensitivity while using Droslyn, requiring closer monitoring.
Overdose
There have been no reports of serious effects from Droslyn overdose. However, symptoms may include nausea, vomiting, or vaginal bleeding. Treatment is symptomatic, with monitoring of potassium and sodium levels, as well as metabolic acidosis in severe cases.
Therapeutic Class
Oral contraceptives
Storage Conditions
Store Droslyn at temperatures below 30°C in a dry place, away from light, and out of the reach of children.










Reviews
There are no reviews yet.