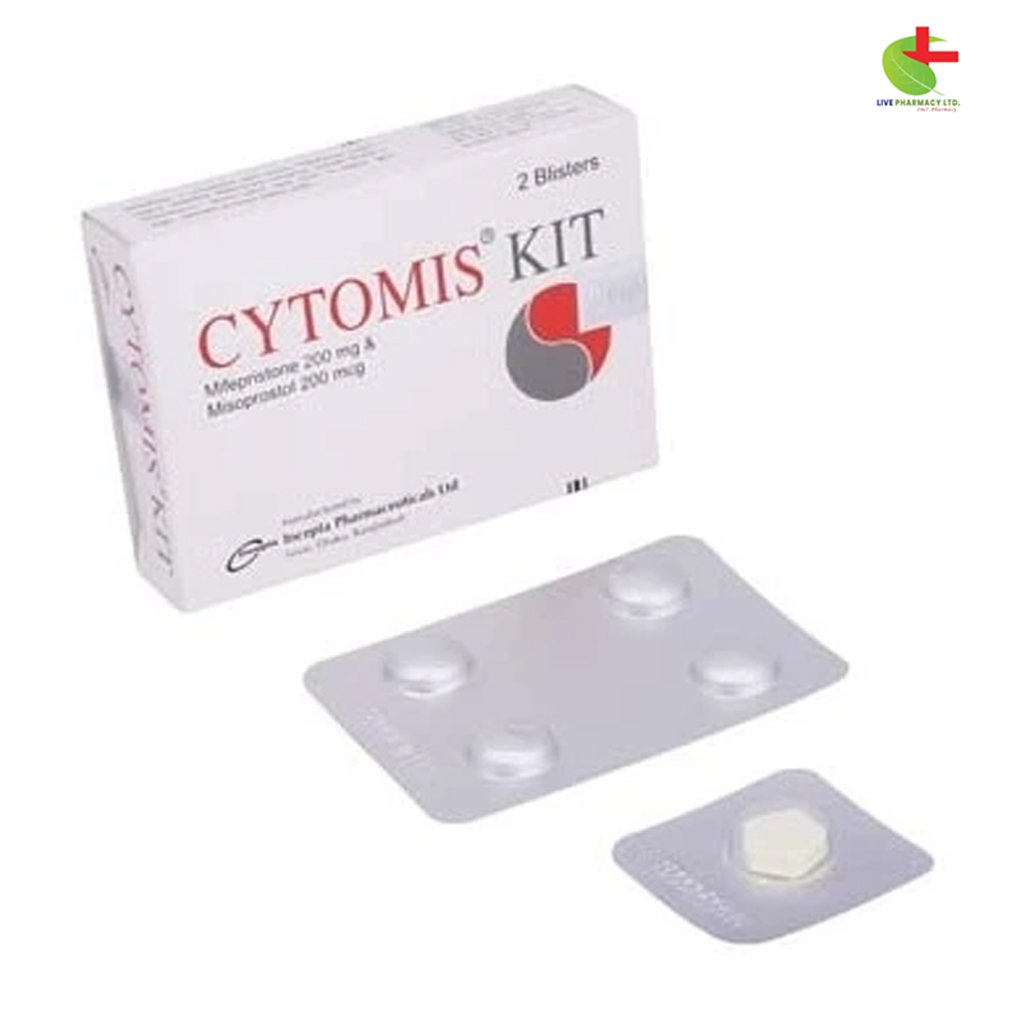
Cytomis Kit
300.00৳ Strip
- Hypnofast is a fast-acting sleep aid designed for the short-term treatment of insomnia and premedication sedation before surgical or diagnostic procedures.
- Its formulation combines Mifepristone and Misoprostol, working together to regulate menstrual cycles or terminate early pregnancies safely.
- Administered under medical supervision, Hypnofast provides effective relief from anxiety and promotes restful sleep, making it a reliable option for those in need of immediate assistance.
- Always consult a healthcare professional for guidance.
 Brand
Brand
|
Incepta Pharmaceuticals Ltd |
|---|---|
 Generics
Generics
|
Mifepristone + Misoprostol |
 Type
Type
|
Tablet |
Indications
This kit is designed for early menstrual regulation (MR) or termination of pregnancy up to 9 weeks (63 days) of gestation.
- Consult a registered healthcare professional for medication guidance.
Composition
Each kit contains two blister strips:
- One strip with 1 tablet of Mifepristone (200 mg).
- Another strip with 4 tablets of Misoprostol (200 micrograms each).
Pharmacology
Mifepristone: A synthetic steroid that blocks progesterone at its receptor sites, inhibiting its effects and facilitating menstrual regulation. It enhances uterine responsiveness to prostaglandins during pregnancy.
Misoprostol: A synthetic analog of prostaglandin E1 that promotes uterine contractions by activating specific receptors, leading to cervical softening and uterine expulsion of contents.
Dosage & Administration
This medication must only be prescribed by qualified healthcare professionals who can assess gestational age and diagnose ectopic pregnancies. Proper medical facilities should be available for emergency care.
- Day 1 (First visit): Administer one tablet of Mifepristone (200 mg) under medical supervision.
- Day 2 (Second visit): 24-48 hours later, take four 200 microgram tablets (800 micrograms) of Misoprostol buccally or sublingually.
- Day 10 to 14 (Third visit): Return for a follow-up examination to confirm complete termination of pregnancy.
- Consult a registered healthcare professional for medication guidance.
Interaction
Mifepristone: Potential interactions with drugs like ketoconazole and grapefruit juice may increase serum levels.
Misoprostol: Does not significantly affect the absorption or efficacy of aspirin in rheumatoid arthritis treatment.
Contraindications
Mifepristone should not be used in individuals with:
- Known hypersensitivity to Mifepristone or Misoprostol.
- Ectopic pregnancy or undiagnosed adnexal mass.
- An IUD in place or certain adrenal and bleeding disorders.
- Inadequate access to emergency medical care.
Side Effects
Common side effects include nausea, vomiting, and diarrhea, along with potential cramping and pelvic pain. Rare effects may include headache and dizziness.
Pregnancy & Lactation
Pregnancy: Mifepristone is only indicated for MR within 63 days. Ongoing pregnancies post-treatment pose risks of fetal malformation, requiring surgical intervention.
Lactation: It is unclear if Mifepristone is excreted in breast milk. Caution is advised, as Misoprostol may cause diarrhea in nursing infants.
Precautions & Warnings
Do not share this medication with others, as it is prescribed for individual conditions. Ensure any IUD is removed before starting treatment. Surgical options should be considered if MR fails.
Use in Special Populations
Hepatic Impairment: Dose adjustments may be needed for patients with liver disease.
Renal Impairment: No routine adjustments are recommended, but doses may need reduction if not tolerated.
Overdose Effects
Mifepristone: High doses generally do not lead to serious adverse reactions, but observe for adrenal failure.
Misoprostol: Symptoms of overdose may include sedation, tremors, and hypotension. Supportive therapy is recommended, as dialysis is unlikely to be effective.
Therapeutic Class
Uterine-active drugs, Prostaglandin analogs.
Storage Conditions
Store in a cool, dry place, away from light.










Reviews
There are no reviews yet.