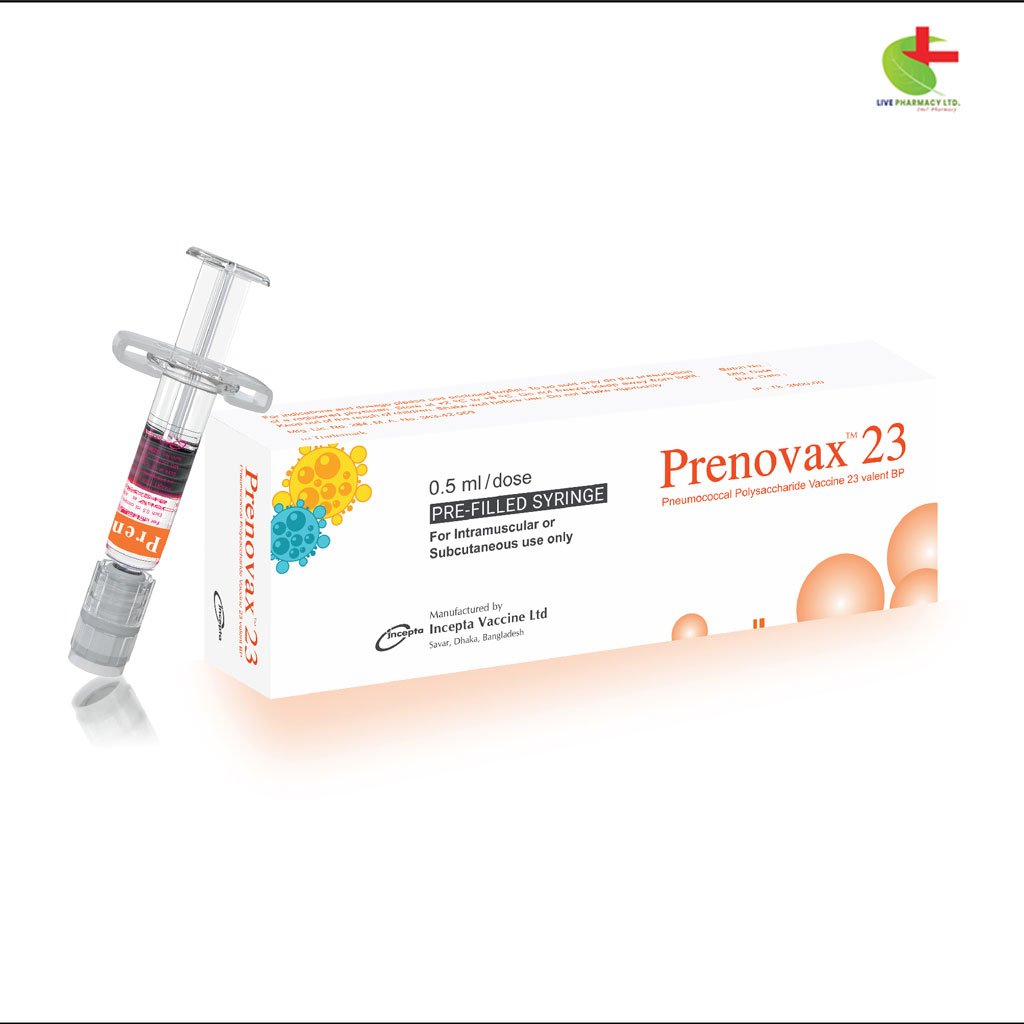
Prenovax 23
2,500.00৳ Syringe(0.5ml)
- Active immunization against pneumococcal disease for individuals aged 2 years and older.
- Contains 23 pneumococcal serotypes, covering approximately 90% of invasive infections.
- Administered via a single intramuscular or subcutaneous injection.
- Recommended for high-risk individuals and those undergoing chemotherapy or immunosuppressive treatments.
- Store refrigerated and protect from light.
 Brand
Brand
|
Incepta Pharmaceuticals Ltd |
|---|---|
 Generics
Generics
|
Polysaccharide Pneumococcal Vaccine |
 Type
Type
|
IM/SC Injection |
Indications
Each 0.5 mL dose of this vaccine provides 25 micrograms of pneumococcal polysaccharide from 23 serotypes: 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19F, 19A, 20, 22F, 23F, and 33F. This vaccine is recommended for the active immunization of children aged 2 years and older, adolescents, and adults to help protect against pneumococcal disease.
Use as directed by a registered healthcare professional.
Pharmacology
This vaccine is derived from purified pneumococcal capsular polysaccharide antigens sourced from 23 different serotypes, responsible for approximately 90% of invasive pneumococcal infections. Included serotypes: 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19F, 19A, 20, 22F, 23F, and 33F.
Dosage & Administration Primary Vaccination:
- Adults and children aged 2 years or older: Administer 0.5 mL via intramuscular or subcutaneous injection.
- This vaccine is not recommended for children under 2 years due to insufficient evidence regarding its safety and efficacy.
Special Dosing Recommendations:
- Pre-Splenectomy or Immunosuppressive Treatment: Administer the vaccine at least two weeks before elective splenectomy or the initiation of chemotherapy/immunosuppressive therapies. Avoid vaccination during chemotherapy or radiation therapy.
- Post-Chemotherapy/Radiation: Immune responses may be reduced after chemotherapy. Wait at least three months after treatment completion before administering the vaccine, with a longer interval considered for intensive or prolonged therapy.
- HIV-Infected Individuals: Vaccination should be done as soon as possible after a confirmed diagnosis.
Revaccination:
- Administer one dose of 0.5 mL via intramuscular or subcutaneous injection.
- Revaccination should follow official recommendations, with a minimum interval of three years. Revaccination within three years is generally avoided due to the potential for increased side effects.
- For Adults: Revaccination may be considered for high-risk individuals after five years or if there is rapid antibody decline. Asplenics and individuals with high-risk conditions may receive revaccination after three years.
- For Children: Routine revaccination is not recommended, except for those at high risk or children aged 10 and older.
Interaction
The pneumococcal vaccine can be administered alongside the influenza vaccine, provided different injection sites and needles are used for each.
Contraindications
This vaccine should not be given to:
- Individuals with known allergies to any vaccine component.
- Those experiencing fever, acute infection, or chronic diseases in the acute stage.
- Individuals with uncontrolled epilepsy or progressive neurological diseases.
- Revaccination within 3 years is not advised unless necessary.
Side Effects
Common side effects, occurring in more than 10% of vaccine recipients, include:
- Pain, tenderness, or swelling at the injection site (60.0%).
- Headache (17.6%), erythema (16.4%), fatigue (13.2%), and muscle aches (11.9%).
Pregnancy & Lactation
There is insufficient data on the vaccine’s effects during pregnancy. It should only be used if the potential benefit outweighs the risk to the fetus. The vaccine’s excretion in breast milk is unknown, so caution is advised when administered to nursing mothers. The impact on fertility has not been evaluated.
Precautions & Warnings
- Avoid vaccination during significant febrile illness or active infection. Do not administer intravenously or intradermally.
- Immunosuppressed individuals (e.g., those on chemotherapy) may not respond as effectively to vaccination, and protection against pneumococcal disease may be lower.
- Adequate emergency provisions, such as epinephrine, should be available to manage potential anaphylactic reactions.
- Patients at high risk of severe pneumococcal infection (e.g., asplenics) should be advised to seek prompt antimicrobial treatment for sudden febrile illness.
- This vaccine may not be effective in preventing infections following certain types of head injuries (e.g., basilar skull fractures).
Therapeutic Class
Vaccines, Anti-sera & Immunoglobulin
Storage Conditions
Store in a refrigerated environment between 2°C and 8°C. Keep vials protected from light.










Reviews
There are no reviews yet.