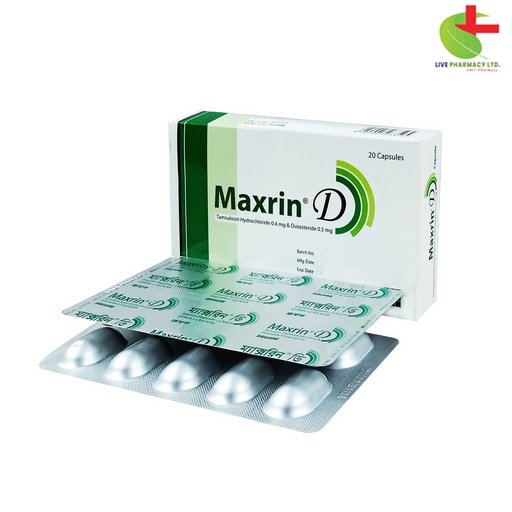
Maxrin D
200.00৳ Strip
- Tamsulosin Hydrochloride & Dutasteride capsule is a combined treatment for moderate to severe symptoms of benign prostatic hyperplasia (BPH).
- It reduces the risk of acute urinary retention and surgery in patients with BPH symptoms.
- Tamsulosin acts on alpha1A-adrenoreceptors, while Dutasteride inhibits 5 alpha reductase, improving urine flow rate and reducing BPH symptoms.
- This medication offers a convenient dosage regimen and comprehensive pharmacological action for effective management of BPH symptoms.
 Brand
Brand
|
Square Pharmaceuticals PLC |
|---|---|
 Generics
Generics
|
Tamsulosin Hydrochloride + Dutasteride |
Indications
Tamsulosin Hydrochloride & Dutasteride capsule effectively addresses:
- Moderate to severe symptoms of benign prostatic hyperplasia (BPH).
- Reduction in the risk of acute urinary retention and the need for surgery in patients with moderate to severe BPH symptoms.
Pharmacology
The combination of Tamsulosin & Dutasteride targets two different mechanisms to alleviate BPH symptoms. Tamsulosin Hydrochloride acts as an antagonist of alpha1A-adrenoreceptors, while Dutasteride inhibits 5 alpha reductase. Together, they improve urine flow rate and alleviate BPH symptoms.
Dosage & Administration
For optimal results, adults (including elderly) are advised to take one capsule (Tamsulosin Hydrochloride 0.4 mg & Dutasteride 0.5 mg) orally approximately 30 minutes after the same meal each day. The capsules should be swallowed whole and not chewed or opened. Renal and hepatic impairment should be considered before dosage adjustments.
Interaction
Limited drug interaction studies are available for Dutasteride-Tamsulosin combination. Caution should be exercised when using together with drugs affecting CYP3A4 metabolism or P-glycoprotein transporters.
Contraindications
The combination is contraindicated in women, children, patients with hypersensitivity to Dutasteride, orthostatic hypotension history, severe hepatic impairment, or peanut/soya allergies.
Side Effects
Common adverse reactions include impotence, decreased libido, breast disorders, ejaculation disorders, and dizziness. Monitoring and evaluation of potential adverse events are recommended.
Pregnancy & Lactation
Avoidance is advised during pregnancy and lactation due to potential risks to the foetus and lack of data on excretion in human milk.
Precautions & Warnings
Careful assessment of benefits versus risks is necessary, especially concerning cardiac failure, effects on PSA levels, prostate cancer risk, renal impairment, hypotension, and Intraoperative Floppy Iris Syndrome.
Overdose Effects
Limited data is available for overdose effects. Symptomatic and supportive treatment is recommended in suspected cases, with close monitoring of vital signs.
Therapeutic Class
The combination belongs to the therapeutic class of BPH/Urinary retention/Urinary incontinence medications.
Storage Conditions
Store in a cool, dry place, protected from light.










Reviews
There are no reviews yet.